When mCH4 = 16.043g of methane gas (CH4) reacts with mo, = 63.996g of oxygen gas (O2), the result is 36.03g of water vapor (H2O) and mco, = 44.009g of carbon combustion (burning) that produces MH20 = dioxide (CO2). a) What is the mass of carbon dioxide (your carbon footprint) produced by burning 1000g of methane? b) When 50g of methane reacts with 150g of oxygen, which gas is left over? How much mass of the remaining gas is left over?
When mCH4 = 16.043g of methane gas (CH4) reacts with mo, = 63.996g of oxygen gas (O2), the result is 36.03g of water vapor (H2O) and mco, = 44.009g of carbon combustion (burning) that produces MH20 = dioxide (CO2). a) What is the mass of carbon dioxide (your carbon footprint) produced by burning 1000g of methane? b) When 50g of methane reacts with 150g of oxygen, which gas is left over? How much mass of the remaining gas is left over?
Chapter9: Chemical Quantities
Section: Chapter Questions
Problem 55A
Related questions
Question
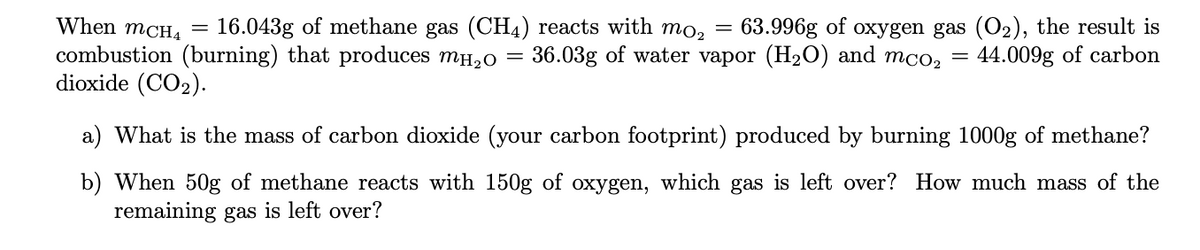
Transcribed Image Text:When mCH4 =
16.043g of methane gas (CH4) reacts with mo, = 63.996g of oxygen gas (O2), the result is
44.009g of carbon
combustion (burning) that produces mH,0 = 36.03g of water vapor (H2O) and
dioxide (CO2).
mCO2
a) What is the mass of carbon dioxide (your carbon footprint) produced by burning 1000g of methane?
b) When 50g of methane reacts with 150g of oxygen, which gas is left over? How much mass of the
remaining gas is left over?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

World of Chemistry
Chemistry
ISBN:
9780618562763
Author:
Steven S. Zumdahl
Publisher:
Houghton Mifflin College Div

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

World of Chemistry
Chemistry
ISBN:
9780618562763
Author:
Steven S. Zumdahl
Publisher:
Houghton Mifflin College Div

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning