when octane (C8H18) is burned under standard conditions, the enthalpy of combustion is -5698.34KJ / mol. Using the information provided. what is the heat of formation of octane?
when octane (C8H18) is burned under standard conditions, the enthalpy of combustion is -5698.34KJ / mol. Using the information provided. what is the heat of formation of octane?
Organic Chemistry
8th Edition
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Chapter2: Alkanes And Cycloalkanes
Section: Chapter Questions
Problem 2.57P: Following are heats of combustion per mole for methane, propane, and 2,2,4-trimeth-ylpentane. Each...
Related questions
Question
when octane (C8H18) is burned under standard conditions, the enthalpy of combustion is -5698.34KJ / mol. Using the information provided. what is the heat of formation of octane?
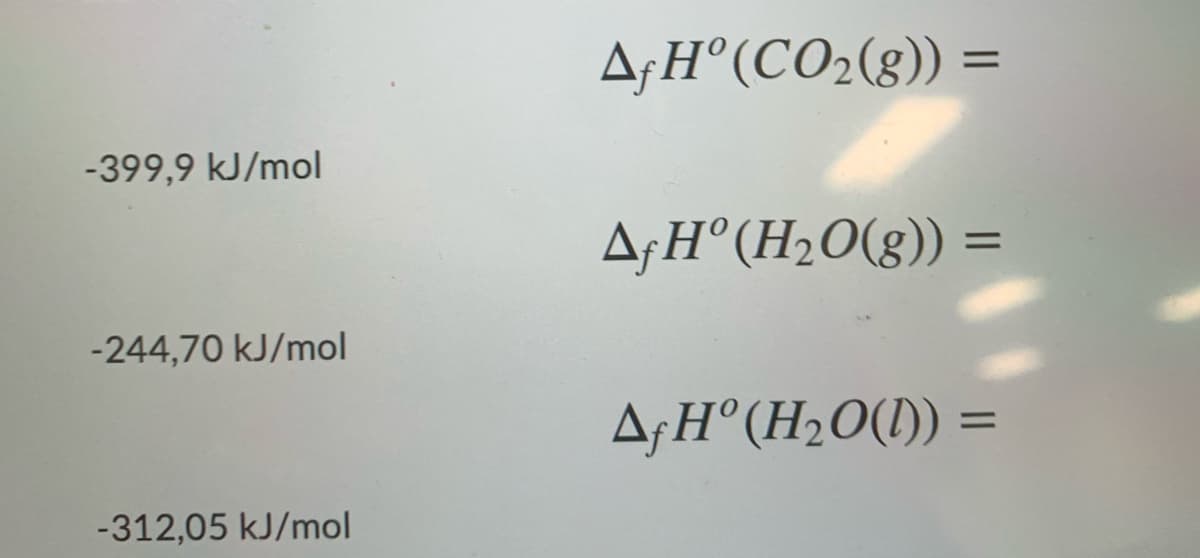
Transcribed Image Text:A;H°(CO2(g)) =
-399,9 kJ/mol
A;H°(H2O(g)) =
%3D
-244,70 kJ/mol
A¡H°(H2O(1)) =
-312,05 kJ/mol
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Recommended textbooks for you

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning