When the Ag* concentration is 1.42 M, the observed cell potential at 298K for an electrochemical cell with the following reaction is 2.079V. What is the Mn2+ concentration? 2Ag*(aq) + Mn(s) 2Ag(s) + Mn²*(aq) Answer: M
When the Ag* concentration is 1.42 M, the observed cell potential at 298K for an electrochemical cell with the following reaction is 2.079V. What is the Mn2+ concentration? 2Ag*(aq) + Mn(s) 2Ag(s) + Mn²*(aq) Answer: M
Chemistry: Principles and Practice
3rd Edition
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Chapter18: Electrochemistry
Section: Chapter Questions
Problem 18.69QE: What is the voltage of a concentration cell of Fe2+ ions where the concentrations are 0.0025 and...
Related questions
Question
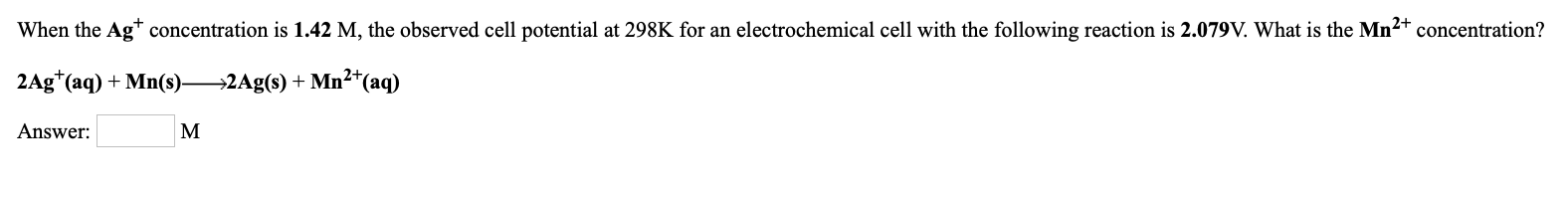
Transcribed Image Text:When the Ag* concentration is 1.42 M, the observed cell potential at 298K for an electrochemical cell with the following reaction is 2.079V. What is the Mn2+ concentration?
2Ag*(aq) + Mn(s) 2Ag(s) + Mn²*(aq)
Answer:
M
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps with 3 images

Recommended textbooks for you

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning


Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning


Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning
