When the nuclear reaction represented by Equation 43.29 is endothermic, the reaction energy Q is negative. For the reaction to proceed, the incoming particle must have a minimum energy called the threshold energy, E. Some fraction of the energy of the incident particle is transferred to the compound nucleus to conserve momentum. There- fore, E, must be greater than Q (a) Show that E.--e(1+) M -Q[1+ Mx a (b) Calculate the threshold energy of the incident alpha particle in the reaction He + 4N 70 + }H a + X → Y + b (43.29)
When the nuclear reaction represented by Equation 43.29 is endothermic, the reaction energy Q is negative. For the reaction to proceed, the incoming particle must have a minimum energy called the threshold energy, E. Some fraction of the energy of the incident particle is transferred to the compound nucleus to conserve momentum. There- fore, E, must be greater than Q (a) Show that E.--e(1+) M -Q[1+ Mx a (b) Calculate the threshold energy of the incident alpha particle in the reaction He + 4N 70 + }H a + X → Y + b (43.29)
Related questions
Question
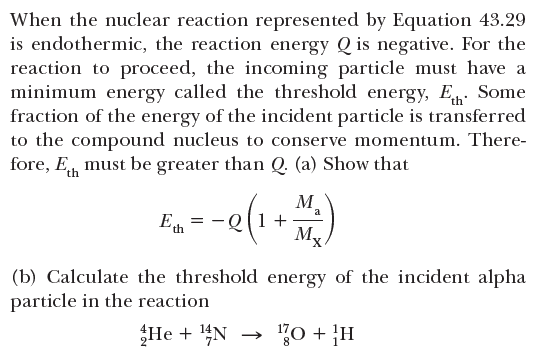
Transcribed Image Text:When the nuclear reaction represented by Equation 43.29
is endothermic, the reaction energy Q is negative. For the
reaction to proceed, the incoming particle must have a
minimum energy called the threshold energy, E. Some
fraction of the energy of the incident particle is transferred
to the compound nucleus to conserve momentum. There-
fore, E, must be greater than Q (a) Show that
E.--e(1+)
M
-Q[1+
Mx
a
(b) Calculate the threshold energy of the incident alpha
particle in the reaction
He + 4N
70 + }H

Transcribed Image Text:a + X → Y + b
(43.29)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 5 steps with 5 images
