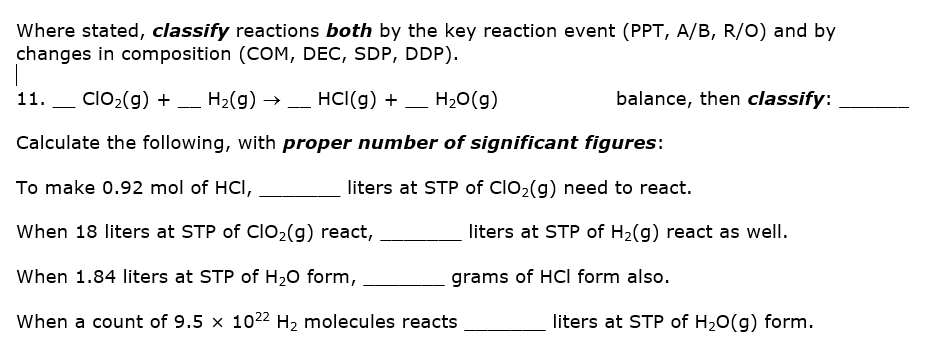
Where stated, classify reactions both by the key reaction event (PPT, A/B, R/O) and by changes in composition (COM, DEC, SDP, DDP). 11. ClO2(g) + – H2(g) → HCI(g) + _ H20(g) balance, then classify: Calculate the following, with proper number of significant figures:
Q: What is the sum of coefficients from the following reaction (both products and reactants)? X2(CO3)3…
A: Sum coefficients can be find out from the balanced reaction.
Q: GRAVIMETRIC ANALYSIS OF A TWO COMPONENT MIXTURE A mixture of NaHCO3 and Na2CO3 reacts with…
A: A numerical problem based on mole concept, which is to be accomplished.
Q: 3. When solutions containing 1.0000 g of BaCl2 and 1.0000 g of NazSO4 were mixed, solid BaSO4…
A: Note - Since you have posted a question with multiple sub-parts, we are supposed to solve only the…
Q: Calculate the percentage of water in potassium sulfate tetrahydrate. All final answers = SN (when…
A: To calculate it 1st we will have to calculate the molecular wt of the given compound . Then the wt…
Q: 2H*(aq) + Sn(s)–H2(g) + Sn²*(aq) ificant figures during intermediate calculations to avoid round off…
A: The cell reaction is: Sn + 2H+ -----> Sn2+ + H2 We have : E°cell = E°H+/H2 - E°Sn2+/Sn…
Q: A binary mixture contains 56 wt. 6 propane C3H8 (MW = 44 g/mol) and the remainder is butane C4H10…
A: Givenwt% of propane=56It indicates weight of propane=56 gmRemaining percentage contains butane ie…
Q: Balance the chemical reaction. ____Pb(NO3)4 + ____Na3PO4 ---> _____ Pb3(PO4)4 + _____…
A:
Q: PbCO3(s)→PbO(s)+CO2(g) Balance the question then express your answers as integers separated by…
A: Balanced chemical equation:- It may be defined as the chemical equation which has all the atoms in…
Q: но What is the chemical formula for the limiting reactant in the reaction shown? chemical formula:…
A: In the first picture, there are five moles of hydrogen which react with four moles of nitrous oxide.…
Q: Aqueous hydrobromic acid HBr will react with solid sodium hydroxide NaOH to produce aqueous…
A: Given mass of HBr = 36 gram mass of NaOH = 10.9 gram mass of water = ?
Q: Butane (C4H10) plus Oxygen yields carbon dioxide plus water
A: Given compound is butane. Butane reacts with oxygen to form carbon dioxide, water and liberate large…
Q: What is the mass of sucrose in 345 g of 12.0% sucrose solution? answer with correct sig figs in…
A: • We need to calculate the mass of sucrose (solute) in grams
Q: Q6) Given the following set of data concerning standardization of NaOH solution: - mass of KHP…
A:
Q: Aspirin yield You record the following data during the Qualitative and Quantitative Characterization…
A:
Q: Mass of unknown mixture (g) 0.5000 g Mass of NaCI formed (g) 0.4100 g Mass of Na,CO; in mixture (g)…
A: A mixture of NaHCO3 and Na2CO3 reacts with hydrochloric acid solution to produce three common…
Q: In the production of a bottled alcoholic mudslide, 3 ingredients are mixed together and bottled:…
A:
Q: Using the available data in the graph (below), calculate the percent by mass of Potassium Bromide in…
A: Percentage by mass is a method to represent the concentration of any solution, and it s defined as…
Q: Many home barbecues are fueled with propane gas (C3H8). What mass of carbon dioxide is produced upon…
A: Given : Volume of propane = 14.5 L = 14500 mL (since 1 L…
Q: 5. In a flask, you heat a mixture of 735.4 g of sodium nitrate and 700.0 grams of water until all of…
A: Given that, Mass of sodium nitrate dissolved in 700 g hot water = 735.4 g Mass of sodium nitrate…
Q: Balance each of the following chemical equations. (Use the lowest possible coefficients for all…
A: Balanced reaction is the reaction in which number of atoms are equal on reactant as well as product…
Q: Octane burns in air to give carbon dioxide and water. CH,8(g) + 0,(g) → co,(g) + H,0(g) Which of the…
A: We have to balance given reaction.
Q: * Identity of Dehydrated salt (for afterwards) Mass of empty dish 70.874g Mass of dish + hydrated…
A: The number of moles of dehydrated salt is 0.002384 mol. nMnCl2=mMnCl2MMMnCl2=0.3 g125.844…
Q: Use the ΔHof data in Table A4.3 to calculate the value of ΔHorxn for the following chemical…
A: The given reaction is:
Q: Since the pandemic has hit, more people are growing their own gardens and processing the fruits of…
A: Each quart jar requires = 6.05g alum So, 50 quart jars would require = 6.05*50 g = 302.5g alum %…
Q: A mixture of NaHCO3 and Na2CO3 reacts with hydrochloric acid solution to produce three common…
A: We have to find the mass of sodium carbonate and sodium bicarbonate in 0.5g mixture
Q: 3. When solutions containing 1.0000 g of BaCl2 and 1.0000 g of NazSO4 were mixed, solid BaSO4…
A: Given that : The mass of BaCl2 used = 1.000 g The mass of Na2SO4 used = 1.000 g The molar mass of…
Q: What is the symbol for the quantity defined by this expression: - log [H3O+]
A: This expression tells that the blank is the negative logarithm of the hydronium ion concentration.…
Q: mixture of NaHCO3 and Na2CO3 reacts with hydrochloric acid solution to produce three
A: We have to find mass of sodium carbonate and sodium bicarbonate in 0.5 g mixture
Q: Liquid hexane (CH. (CH₂(CH₂) CH₂) reacts with gaseous oxygen gas (O₂) to produce gaseous carbon…
A:
Q: Mass of Na,CO, in mixture (g) Mass of NaHCO, in mixture (g) % Na,CO3 % NaHCO,
A:
Q: For a 77.1% mixture of methanol (CH3OH) in water on a mass basis, calculate the percentage of…
A: The number of moles of a compound is related to its mass by the following relation: Number of moles…
Q: Gypsum is the hydrate of calcium sulfate, CaSO4 · 2 H20. It is the primary material in sheet rock,…
A: Hydrated crystal contains certain number of water molecules. When hydrated crystal is heated these…
Q: Liquid octane (CH3(CH2) CH3) reacts with gaseous oxygen gas (02) to produce gaseous carbon dioxide…
A: Given: Mass of octane = 11.4 g Mass of O2 = 71.6 g Molar mass of O2 = 32 g/mol Molar mass of octane…
Q: Obtain values from the pictures found in the procedure to complete the data table below. Remember to…
A: “Since you have posted a question with multiple sub-parts, we will solve first three subparts for…
Q: Knowing that 1 calorie = 4.184 joules and 1kWh = 3.6 x 106 Joules, convert the following: 2739…
A: Given: 1 calorie = 4.184 joules 1kWh = 3.6 x 106 Joules Toconvert: 2739 teraWh into nanocalories.
Q: Mass of unknown mixture (g) 0.5000 g Mass of NaCI formed (g) 0.4100 g Mass of Na;CO; in mixture (g)…
A:
Q: Carbon dioxide (CO2) is the gas that is mainly responsible for global warming (the greenhouse…
A:
Q: volume
A:
Q: Aqueous hydrochloric acid (HCI) reacts with solid sodium hydroxide (NaOH) to produce aqueous sodium…
A: Given: Mass of HCl = 2.6 g Mass of NaOH= 1.8 g Mass of water produced (Actual yield) = 0.640 g…
Q: %3D .1 mL 441.)10 1 dt 10 I mL. %3D 441. 10 101 1°
A: So basically the question have asked to solve the unit conversation.
Q: In the reaction: KClO3(aq) + 5KCl(aq) + 6HNO3(aq) → 6KNO3(aq) + 3Cl2(g) + 3H2O(l) How many grams…
A:
Q: with hydrochloric acid solution to produce three common products, sodium chloride, carbon dioxide…
A: We have to find mass of sodium carbonate and sodium bicarbonate in 0.5 g mixture
Q: Automobiles are often implicated as contributors to global warming because they are a source of the…
A: In a balanced chemical reaction the reactions react and products form in the molar ratio of their…
Q: Aqueous hydrochloric acid (HCL) will react with solid sodium hydroxide (NaOH) to produce aqueous…
A: Given reaction is , acid base reaction which gives salt and water as products. Also, given values…
Q: 2.027g of Salicylic acid is weighed and mixed it with excess acetic anhydride. (Show ALL steps and…
A: Given: Mass of salicylic acid = 2.027 g Known: Molar mass of salicylic acid =138.12g/mol Molar mass…
Q: Mass of unknown mixture (g) 0.5000 g Mass of NaClI formed (g) 0.4100 g Mass of Na;CO, in mixture (g)…
A: Given : Mass of mixture = 0.5000 g And mass of NaCl produced = 0.4100 g The balanced reactions…
Q: Maple syrup is made from the sap of a maple tree. Whe the sap is tapped from the tree it is 4.0% by…
A: Here we are required to find the mass of sap that would be needed for making 1kg of syrup
Q: जिनण्या व जखमल : Fe CNO3),+3 NaoH ज्ठमाक > FECOH)3 + 3 NaNog 3 2.००८ ० १ ०. न30M NqoH Sdcीस emPखग्र…
A: Volume of NaOH = 2.00 L Molarity of NaOH = 0.730 M
Q: 3 Fe (s) + 4 H2O (g) →Δ Fe3O4 (s) + 4 H2 (g) Enter your answer in grams for water into the first…
A: Given mass of Fe3O4 = 375 g Molar mass of Fe = 55.845 g/mol Molar mass of Fe3O4 = 231.533 g/mol…
Q: Aqueous sulfuric acid H2SO4 reacts with solid sodium hydroxide NaOH to produce aqueous…
A: 1 mol of sulfuric acid reacts with 2 mol of sodium hydroxide to give 1 mol of sodium sulfate and…
Step by step
Solved in 2 steps with 2 images

- A) An aqueous solution of sodium nitrate, NaO3, is made by dissolving 21.6 grams of sodium nitrate in sufficient water in a 500. mL volumetric flask, and then adding enough water to fill the flask to the mark. What is the weight/volume percentage of sodium nitrate in the solution? Weight/volume percentage = _______% B) An aqueous solution of ammonium fluoride, NH4F, is made by dissolving 32.4 grams of ammonium fluoride in sufficient water in a 500. mL volumetric flask, and then adding enough water to fill the flask to the mark. What is the weight/volume percentage of ammonium fluoride in the solution? Weight/volume percentage = ________%A 800.0 mL aqueous solution of 93.0 mM CoCl2 is combined with 399.0 mL of aqueous 54.0 mM K3PO4. If the limiting reagent is completed consumed during this reaction, how many grams of solid precipitate will be produced? Express your answer in units of grams using at least three significant figures.The fish canning industry has a waste effluent with the specification as follows: BOD5 = 150 mg/L, COD = 230 mg/L, CaCO3 = 65 mg/L, pH = 7,0, Odor = 11 (TON, where every 1 mg/L ammonia-N equals to 2 TON), Oil = 300 mg/L. Give recommendation of what kind of treatment (physical and chemical steps) is required to treat the wastewater, and why do you select those steps.
- A solution is prepared by dissolving 155.0 mL of methanol (CH3OH, density = 0.791 g/mL) in 250 mL of water (density 1.00 g/mL) to give a final solution volume of 350.0 mL. Calculate the (a) molarity of the solution, (b) %wt/wt, (c) %wt/vol, (d) %vol/volGiven Active Ingredient: precipitated sulfur (ointment) Raw Materials: 500 g calcium polysulphide and 1.5 kg hydrochloric acid Actual Yield: 343.4g precipitated sulfur Formulation: 250 mg per jar Dosage form: Ointment packaging:100 jars per box Synthesis and Packaging (Need answer)- Balanced Chemical Equation:- % composition by mass of each compound:- Mass to Mass Stoichiometry Calculation:- Limiting Reagent:- Excess Reagent:- Amount (g) in excess: % Yield:- Number of dosage form and packaging that can be produced from stoichiometric solution:A 244.5-g sample of ground water is analyzed for calcium. The Ca2+ in the sample is first precipitated and filtered-off as NH4CaPO4.7H20. This precipitate is dried and heated, releasing water and ammonia to yield anhydrous calcium pyrophosphate (CaP2O7). The mass of CaP2O, obtained is 0.0419 g. Give the calcium content of the ground water in parts per million (to three significant figures).
- What is the correct volume of 2.5 M PhMgBr in THF is needed to completely react with 1.5 mL of methyl benzoate? You do not need to consider an excess and should calculate (and choose) the minimum amount as required by the reaction stoichiometry). Enter your answer to one decimal place WITH the proper unit of measurement.GRAVIMETRIC ANALYSIS OF A TWO COMPONENT MIXTURE A mixture of NaHCO3 and Na2CO3 reacts with hydrochloric acid solution to produce three common products, sodium chloride, carbon dioxide and water according to the following balanced chemical equations. NaHCO3(s) + HCl (aq) → NaCl(s) + CO2(g) + H2O(l) (1) Na2CO3(s) + 2HCl(aq) → 2NaCl(s) + CO2(g) + H2O(l) (2) Mass of mixture (gr) = mass (gr) of NaHCO3 + mass (gr) of Na2CO3 (3) Mass of NaCl (gr) = mass (gr) of NaCl formed + mass (gr) of NaCl formed (4) from the NaHCO3 from the Na2CO3 NOTE!!! Fill-out the tabulation below. Present the complete solution for the determination of the unknown amounts.In the standardization of HCl using pure anhydrous sodium carbonate as the primarystandard for methyl orange as an indicator, 1.0 mL HCl was found to be equivalent to 0.05gof sodium carbonate (MW =106). The normality of HCl is:
- An unknown sample containing citric acid (C6H8O7) was analyzed using 0.2347N NaOH. The following data were obtained in one of the trials of the analysis: Mass of Unknown 0.4232 g Initial reading of NaOH 2.50 mL Final reading of NaOH 28.00 mL Write the balanced chemical equation involved. What is the net volume of NaOH reacted? What is the molar mass of citric acid? Calculate % citric acid in the sample.An aqueous solution of potassium sulfate, K2SO, is made by dissolving 27.6 grams of potassium sulfate in sufficient water in a 300. mL volumetric flask, and then adding enough water to fill the flask to the mark. What is the weight/volume percentage of potassium sulfate in the solution?Weight/volume percentage = %Zinc and magnesium react with hydrochloric acid to produce the metal chlorides and hydrogen gas. A 10.00 gram smaple of a mixture of Zn and Mg was with the stoichiometric quanitity of HCl. The reaction mixture was then reacted with 156 mL of 3.00M silver nitrate to produce the maximum quantity of silver chloride. First determine the % magnesium in the mixture- then, if 76.0 mL of HCl was added, what was the molarity of the HCl?

