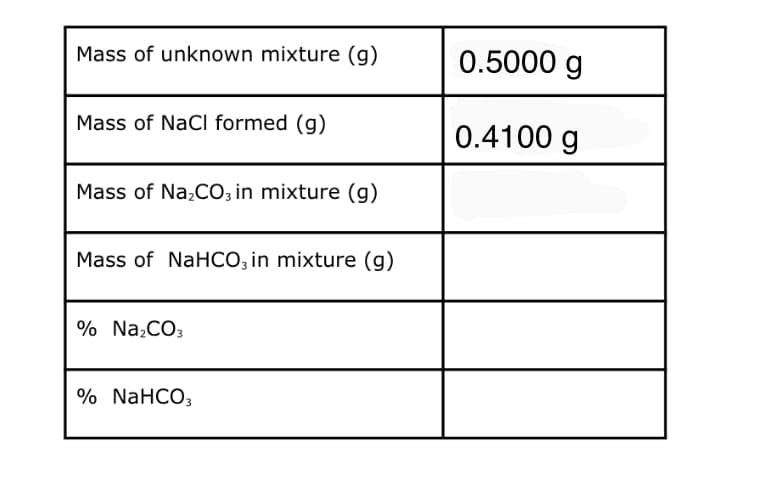
Mass of unknown mixture (g) 0.5000 g Mass of NaCI formed (g) 0.4100 g Mass of Na,CO; in mixture (g) Mass of NaHCO, in mixture (g) % Na,CO3 % NaHCO,
GRAVIMETRIC ANALYSIS OF A TWO COMPONENT MIXTURE
A mixture of NaHCO3 and Na2CO3 reacts with hydrochloric acid solution to produce three common products, sodium chloride, carbon dioxide and water according to the following balanced chemical equations.
NaHCO3(s) + HCl (aq) → NaCl(s) + CO2(g) + H2O(l) (1)
Na2CO3(s) + 2HCl(aq) → 2NaCl(s) + CO2(g) + H2O(l) (2)
Mass of mixture (gr) = mass (gr) of NaHCO3 + mass (gr) of Na2CO3 (3)
Mass of NaCl (gr) = mass (gr) of NaCl formed + mass (gr) of NaCl formed (4)
from the NaHCO3 from the Na2CO3
NOTE!!! Fill-out the tabulation below. Present the complete solution for the determination of the unknown amounts.
Trending now
This is a popular solution!
Step by step
Solved in 2 steps









