Which class of molecules listed below does not allow for hydrogen bonding to occur with carboxylic acids? O aldehydes O alkanes O ethers O all of these
Which class of molecules listed below does not allow for hydrogen bonding to occur with carboxylic acids? O aldehydes O alkanes O ethers O all of these
Chemistry for Today: General, Organic, and Biochemistry
9th Edition
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Chapter11: Organic Compounds: Alkanes
Section: Chapter Questions
Problem 11.28E: Classify each of the following compounds as a normal alkane or a branched alkane: a. b. c. d. e. f.
Related questions
Question
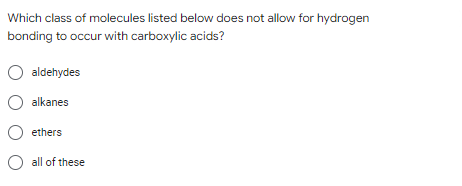
Transcribed Image Text:Which class of molecules listed below does not allow for hydrogen
bonding to occur with carboxylic acids?
O aldehydes
O alkanes
O ethers
O all of these
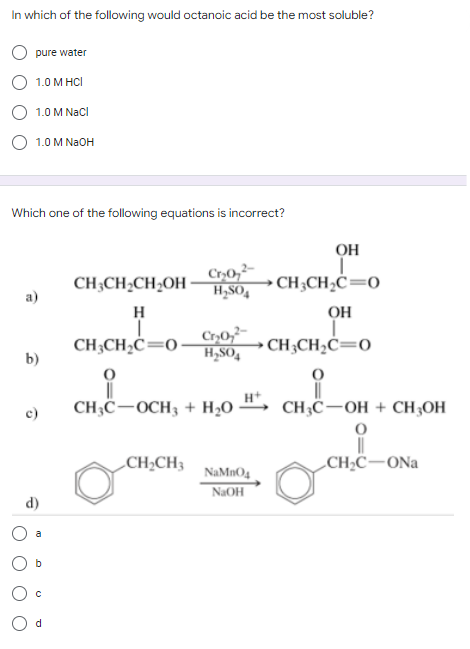
Transcribed Image Text:In which of the following would octanoic acid be the most soluble?
pure water
1.0 M HCI
1.0 M NaCl
1.0 M NaOH
Which one of the following equations is incorrect?
CH3CH₂CH₂OH
Cr₂0₂²
H₂SO4
a)
H
CH3CH₂C=0-
Cr₂0₂2
H₂SO4
b)
CH,COCH3 + H2O
CH₂CH3
c)
d)
b
с
d
NaMnO4
NaOH
ОН
CH3CH₂C=0
ОН
CH3CH₂C=0
CH3C-OH + CH3OH
O
CH₂C-ONa
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning


Organic And Biological Chemistry
Chemistry
ISBN:
9781305081079
Author:
STOKER, H. Stephen (howard Stephen)
Publisher:
Cengage Learning,

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning


Organic And Biological Chemistry
Chemistry
ISBN:
9781305081079
Author:
STOKER, H. Stephen (howard Stephen)
Publisher:
Cengage Learning,

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co