Which element is reduced in this reaction? 2Cr(OH)3 + 3OCI + 40H¯→2CrO4-2 +3C1 + 5H20 Enter the chemical symbol of the element. • View Available Hint(s) is reduced Submit
Which element is reduced in this reaction? 2Cr(OH)3 + 3OCI + 40H¯→2CrO4-2 +3C1 + 5H20 Enter the chemical symbol of the element. • View Available Hint(s) is reduced Submit
Chemistry: An Atoms First Approach
2nd Edition
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Steven S. Zumdahl, Susan A. Zumdahl
Chapter19: The Representative Elements
Section: Chapter Questions
Problem 74AE: The inert-pair effect is sometimes used to explain the tendency of heavier members of Group 3A (13)...
Related questions
Question
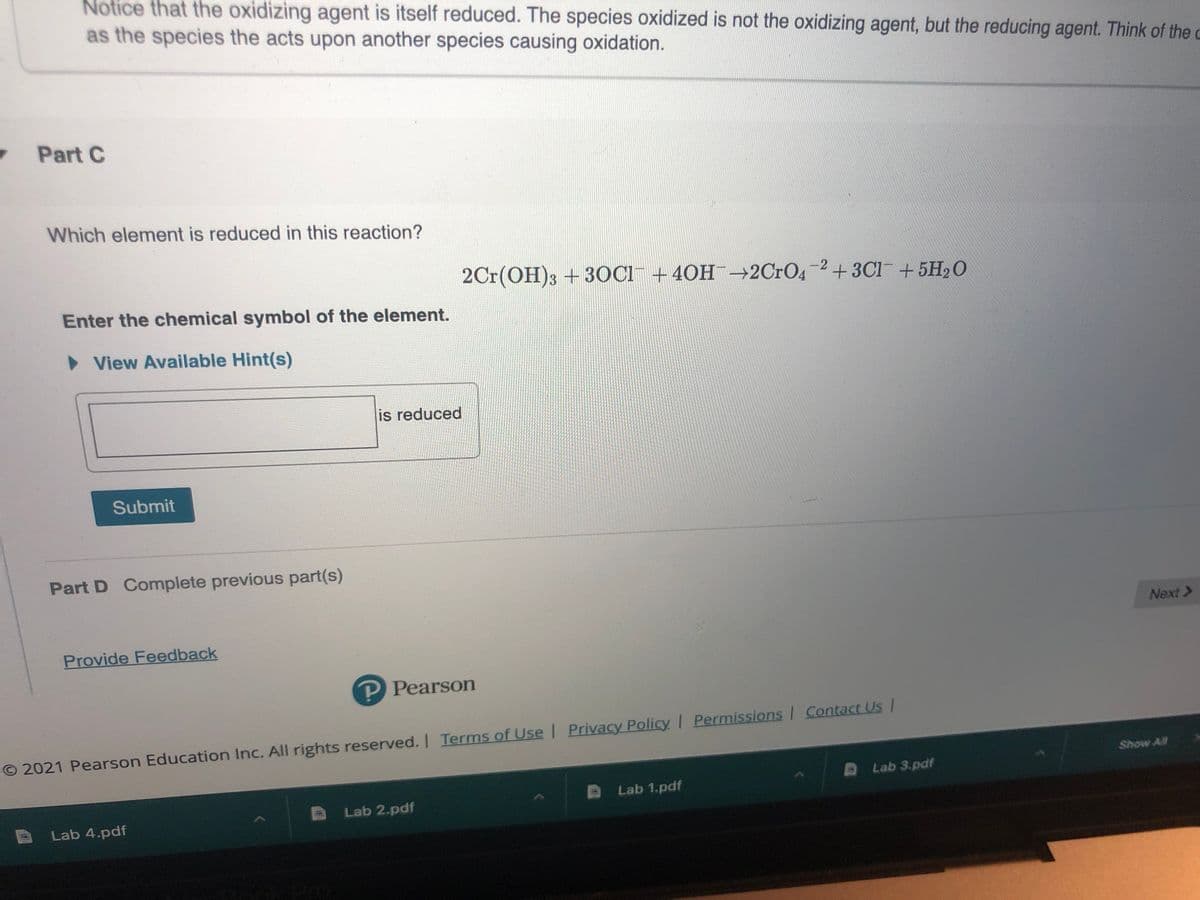
Transcribed Image Text:Notice that the oxidizing agent is itself reduced. The species oxidized is not the oxidizing agent, but the reducing agent. Think of the c
as the species the acts upon another species causing oxidation.
Part C
Which element is reduced in this reaction?
2Cr(OH)3 + 3OC1¯ +40H¯-2C1O4-2+3C1+5H2O
Enter the chemical symbol of the element.
• View Available Hint(s)
is reduced
Submit
Part D Complete previous part(s)
Next>
Provide Feedback
P Pearson
O 2021 Pearson Education Inc. All rights reserved. I Terms of Use | Privacy Policy Permissions Contact Us/
Show All
Lab 3.pdf
Lab 1.pdf
Lab 2.pdf
Lab 4.pdf
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Recommended textbooks for you

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax