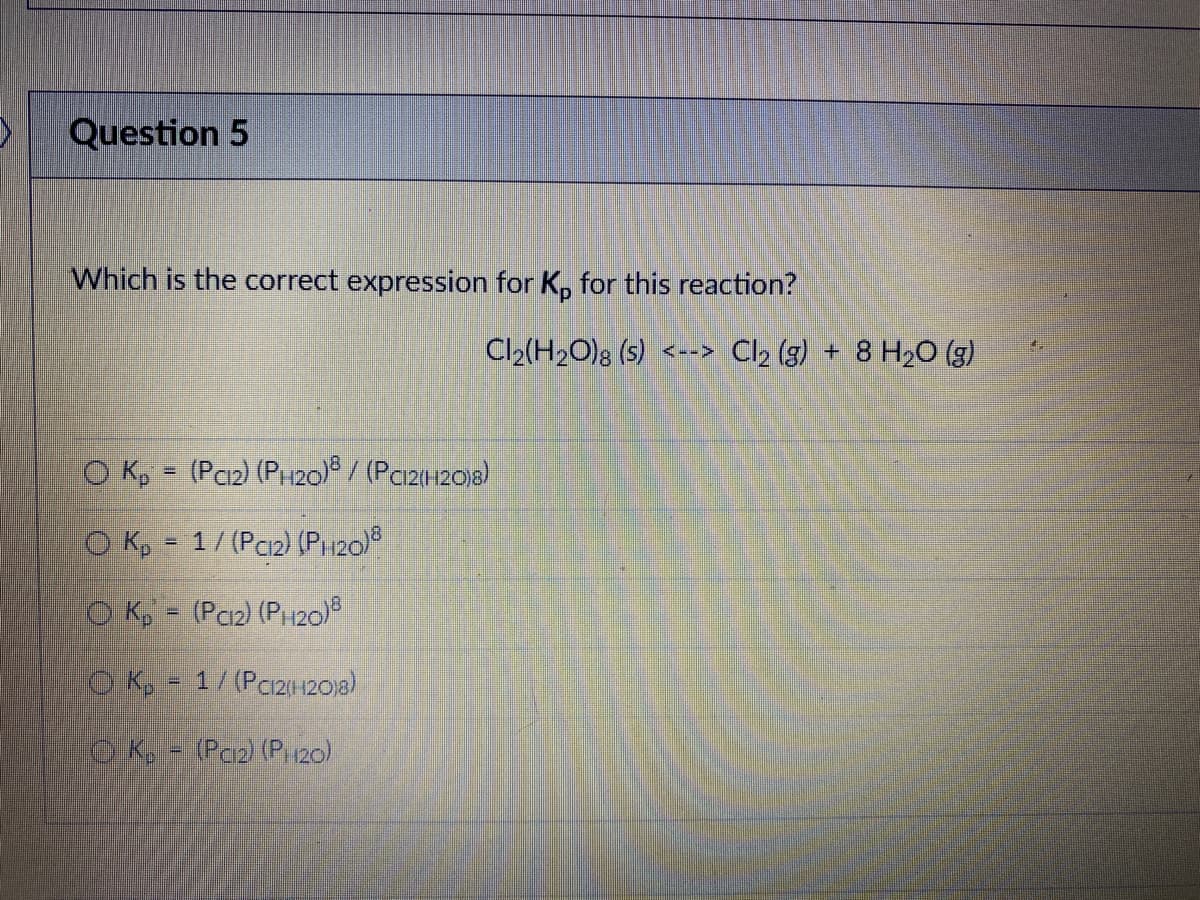
Which is the correct expression for K, for this reaction? Cl2(H20); (s) <--> Cl2 (g) + 8 H2O (g) O K, = (Pci2) (PH20) / (Paz120)8) O Kp = 1/ (Pci2) (PH20) O K, (Pc2) (P120)8 !! O K, - 1/(Pc2120)8) OK - (Paz) (Pi120)
Q: Consider the following reaction where Kc = 7.00 × 10-5 at 673 K. NH4I(s) NH3(g) + HI(g) A reaction…
A: For this reaction ; Qc = [NH3]×[HI]/[NH4I] And we know ; If Qc>Kc the reaction will proceed…
Q: For the reaction N2O4(g)⇌2NO2(g)N2O4(g)⇌2NO2(g), the value of K at 25∘C25∘C is 7.19×10−37.19×10−3.…
A: This question is based on equilibrium concepts. For this question one needs to know how to write the…
Q: At 500 K, the reaction PCl5(g) ⇌ PCl3(g) + Cl2(g) hasKp = 0.497. In an equilibrium mixture at 500 K,…
A: The given balanced equation for the reaction would be PCL5g⇌PCL3g+CL2g
Q: Consider the following reaction where K. = 9.52×10-2 at 350 K. CH4(g) + CCI4(g) 2CH2CI2(g) A…
A:
Q: . Write equilibrium constant expressions for the following reactions. a. N2O4(g) -> NO2(g) b.…
A: Equilibrium constant expression can be written as concentrations of the products raised to their…
Q: At a given temperature, K for the reaction 2HCI (g) → H₂ (g) + Cl₂ (g) is 4.17x10-34. What is K for…
A:
Q: At 500 K the reaction PCl5(g) → PCl3(g) + Cl2(g) has Kp = 0.497. In an equilibrium mixture at 500 K,…
A: The given balanced equation for the reaction is,
Q: 3. A sealed vessel contains 0.25 M COC12, 0.11 M CO, and 0.11 M Cl, at 668 K, which is an…
A:
Q: For the hypothetical reaction AX2 <--> A + X2 Kc = 1.50 If the reaction container…
A: For hypothetical reaction, Equilibrium constant(Kc) equals to ratio of concentration of products…
Q: Suppose sulfur dioxide reacts with oxygen at 25°C. 2802 (9) + O2(9) → 28O3(9) The equilibrium…
A: Reactions' extent is governed by an important temperature dependent quantity referred as equilibrium…
Q: 21. For the equilibrium, H2(g)+I2(g) ⇄2HI(g), what is K if at equilibrium, [H2]=[I2]=0.0100 M and…
A: Given equilibrium reaction H2g+I2(g)⇆2HIg Equilibrium concentration of H2=I2=0.0100 MHI=0.0317 M
Q: Suppose a mixture was prepared containing 0.0100 mol H2, 0.0150 mol F2, and 0.0180 mol HF in a 1.25…
A: Equilibrium is a condition at which both forward and reverse reactions are taking place with no…
Q: 12. 15.70 An equilibrium mixture of N2, H,, and NH3 at 700 K con- tains 0.036 M N, and 0.15M H2. At…
A: The equilibrium constant in terms of concentration represents the relation between concentration of…
Q: At -16.2 °C the concentration equilibrium constant K-9.7 x 105 for a certain reaction. Here are some…
A: Here at -16.2°C the equilibrium constant Kc of a reaction is 9.7×10-5.At constant pressure the…
Q: Numeric At 298 K the reaction N2 (g) + 3 H2 (g) 2 NH3(g) has K, = 4.2 x 108. What is the value of K.…
A: Applying concept of relationship between chemical constants of different type of reaction.
Q: Consider the following system at equilibrium where AH° = 18.8 kJ/mol, and Kc = 9.52x10-², at 350 K.…
A:
Q: At 1100 K, K, = 0.18 for the reaction 2SO2 (9) + O2(g) 2803 (g) What is the value of K at this…
A:
Q: Be sure to answer all parts. The equilibrium constant (K) for the reaction 2HCI(g) - H,(g) + Cl,(g)…
A: What is the equilibrium constant of the required reaction---
Q: A favorite reaction is calcite dissolution: CACO36) -→ Ca?+ + CO??- K = 10-83 Will the solid form or…
A: ANSWER : (a) Solid will be formed as we approach vent deep in the pacific Ocean. (b) Concentration…
Q: Equilibrium. (2) Write the Ke expression for the following balanced equilibrium reaction. N2 (g) + 3…
A: The reaction given is hence the equilibrium constant expression is where [H2 ] = equilibrium…
Q: 3. An antibody (B) binds to an antigen (G) to form (BG product and reaches equilibrium. At…
A: The explanation is given below-
Q: Consider the following reaction where K, = 10.5 at 350 K. 2CH2CI2(g) CH4(9) + CCI4(g) A reaction…
A:
Q: What is the Kp expression for the reaction: + 4 HCI9) → CH4(g) + 2 Cl2(g) OKp = [CH,][Cl,]² (HC114…
A:
Q: Dinitrogen tetroxide gas, dissociates at room temperature to give nitrogen dioxide gas. a) The…
A: Kc= product/reactant Percent of Dissociation is 91% N2O4 ⇌2…
Q: For the reaction N2O4(g)⇌2NO2(g)N2O4(g)⇌2NO2(g), the value of K at 25∘C25∘C is 7.19×10−37.19×10−3.…
A:
Q: Consider the following reaction where K. = 1.80×102 at 698 K. 2HI(g) H2(g) + I2(g) A reaction…
A: The ratio of the products of the molar concentration of gaseous products to that of the product of…
Q: see photo. Problem # 15.18
A: If the equilibrium concentration or pressure is greater than one, then the reaction favors the…
Q: Write the equilibrium expression for the following reaction. H2SO (aq) + 2KOH(aq) 2H20(1) +…
A: General equilibrium constant is as: A + 2B ⇌ C + 3D Then, Keq = productreactant Equilibrium…
Q: At 500 K, the reaction PCl5(g) <=> PCl3(g) + Cl2(g) has Kp = 0.497. In an equilibrium mixture…
A:
Q: Consider the following reaction: cocCl2(g) co(g) + Cl2(g) If 9.25x10-3 moles of COCI,(g), 0.607…
A:
Q: 3. For the reaction, PClsia) PClaia) + Clhia) , Kea = 33.3 at 760°C. Is this system at equilibrium…
A: The equilibrium constant can be written as the ratio of concentration of products to the…
Q: The value of K, for the reaction is 10.9 at 525.4 °C. What is the value of K, at 525.4 °C?
A:
Q: For the system: 2 HI(g) = H2(g) + 12(g), at 445°C, the value for K, is 0.020. A mixture of H2, 12,…
A: The given equilibrium is 2 HI (g) <--------> H2 (g) + I2 (g) The equilibrium constant is K…
Q: 6.Given the following information: 2A(g) + B(g) A2B (g) 2A(g) + C2lg) 2AC(g) Kp2 (3/2)A2(g) + B(g)+…
A: The given equations can be numbered as 1, 2 and 3 as given below:
Q: Suppos constants for the two reactions XeF(g) + H2O(g) = XeOF4(g) + 2 HF(g) XeO4(g) + XeF,(g) 2…
A: Reaction 1: XeF6 (g) + H2O (g) -----XeOF4 (g) + HF (g) .......K1 Reaction 2: XeF4 (g) +…
Q: For the reaction, Fe203 (s) + 3 H2(g) 2 Fe(s) + 3 H20g), AG - 53 k at 25 °C and AH- 100 k). Which…
A:
Q: If you take the following chemical equation: Brze) 2B12) The K, is 8.08 x 10 -4 for the temperature…
A: Br2(g) -------> 2Br(g)
Q: For the reaction below, K = 0.11 at 375°C : ?2 (?)+3 ?2 (?)⇌ 2 ??3 (?) If the initial…
A: For a reaction, A + B = C+D the reaction quotient, Q = [C][D]/[A][B] Now if the value of Q = k then…
Q: Question 15.24
A: Given information:Equilibrium constant for reaction (K) = 1.08×107Temperature (T) = 700 ͦC…
Q: 1a.At 100 C, Kp = 6.5 x 10-2 for the reaction: 2NO2(g) ⇌ N2O4 (g). What is Kc? 1b.For the…
A:
Q: 54. The value of log10 K for a reaction A Bis Given, A,H98 K = - 54.07 kJ mol-1 A,S98 K = 10 JK-1…
A:
Q: For the system: 2 HI(g) = H2(g) + 12(g), at 445°C, the value for K, is 0.020. A mixture of H2, 12,…
A: We will first calculate Qc , then compare with Kc to answer the question.
Q: Help me plsss.. *For the reaction N2(g) + O2(g) ↔ 2 NO(g), Kc = 4.080×10–4 at 2000 K. What is the…
A:
Q: + S2(g) is 2.25 × 10–4. If [H2S] = 4.84 × 10–3
A: To determine the concentration of the [S2] in the reaction. We have the equilibrium constant Kc =…
Q: At 500 K the reaction PCl5(g) → PCl3(g) + Cl2(g) has Kp = 0.497. In an equilibrium mixture at 500 K,…
A: PCI5→PCI3+CI2Kp=4.497Ppci5=0.860 atmPpci3=0.350 atm
Q: The K. for CO (g) + 3 H2 (g) = CH4 (g) + H2O (g) is 9.17 * 10-2. If a 3.5 L vessel is set up with…
A: Normally for those chemical reactions , in which Kc value more than value of Qc , Reaction direction…
Q: In an experiment, gaseous C2H4 and H2O, at 25 mmol each, were mixed in a 50-mL flask that contains…
A: Since you have asked multiple questions, we will solve the first question for you. If youwant any…
Q: At 906.4 °C, the equilibrium constant K, is 4.81 × 10-5. What is the value of K?
A: Given, Kp = 4.81x10-5 Temperature, T = 906.4 °C
Q: 1.4 4 (a) Calculate the value of Ke for the reaction: PCls ( PC13 (8) + Cl2 (g) AH Positive Given…
A:
Q: At –17.9 °C the concentration equilibrium constant K =7.8 × 10 for a certain reaction. Here are some…
A:
Step by step
Solved in 2 steps with 2 images

- What is the equilibrium concentration (in M to three decimal places) of HBr for the following reaction if [H2]i = [Br2]i = 0.500 M at 25 °C? H2(g) + Br2(g) ⇌ 2HBr(g) K=0.0003511.For the aqueous reaction A + B à C + D, the equilibrium concentrations in moles per liter are [A] = 0.002, [B] = 0.005, [C] = 0.00004 [D] = 0.00025 i. What is the equilibrium constant Keq? ii. What is the dissociation constant pK? a. The partial pressure of gas A is 10 ppmv. It has a Henry’s constant for dissolution in water of KH = 0.02Mg3(PO4)2 has Ksp = 1 * 10^-25. Write the balanced equation to which this equilibrium constant corresponds. Does a precipitate form when 200 mL 0.00017 M Mg(NO3)2 are combined with 900 mL 0.00022 M Na3PO4? Support with a calculation.
- Calcium carbonate (e.g. from limestone) is relatively insoluble in water, and the solubilitydecreases with rising temperature. This is why CaCO3 precipitates out as ‘scale’ in hot watermore readily than cold water. At a temperature of 20oC , calcium carbonate pKs = 8 . 8; at35oC , pKs = 9 . 25. If you had water that was at equilibrium with excess calcium carbonate at20oC and then raised the temperature to 35oC , what is the mass of CaCO3 that will precipitateout per litre of water? Hint: the difference in solubility will drive the reaction back to solid. CaCO3 <-> Ca2+ + CO32-In an experiment to study the formation of HI(g), H2(g) + I2(g) ⇌ 2 HI(g) At equilibrium, [H2] = 0.0848, [I2] = 0.0207 and [HI] = 0.074. Calculate the value of Kc. *The answer is 3.12, but I would like an explanation*Calcium carbonate (e.g. from limestone) is relatively insoluble in water, and the solubilitydecreases with rising temperature. This is why CaCO3 precipitates out as ‘scale’ in hot water more readily than cold water. At a temperature of 20oC, calcium carbonate pKs= 8.8; at 35oC, pKs= 9.25. If you had water that was at equilibrium with excess calcium carbonate at 20oC and then raised the temperature to 35oC, what is the mass of CaCO3 that will precipitate out per litre of water? Hint: the difference in solubility will drive the reaction back to solid. CaCO3(s)↔Ca2++CO2−
- If 13.0 mL of 0.19 M MgCl2 are mixed with 21.9 mL of 0.54 M NaOH, what will be the final concentration of Mg2+ in solution when equilibrium is established? Assume the volumes are additive. Ksp = 1.2 ✕ 10-11 for Mg(OH)2. (in M)Give typed solution For each initial mixture (1),(2), and (3) shown below, determine the direction the reaction will proceed to reach equilibriumThe solubility product for Zn(OH)2 is 3.0 * 10-16. The formation constant for the hydroxo complex, Zn(OH)42- , is 4.6 * 1017. What concentration of OH- is required to dissolve 0.015 mol of Zn(OH)2 in a liter of solution?
- What is the concentration of Y at equilibrium (in M) if we start with [X] = 1.80M and [Y] = 0.00 M? In 4 significant figures.At 25 °C, the reactionCaCrO4(s) ⇌ Ca2+(aq) + CrO42-(aq)has an equilibrium constant Kc = 7.1 x 10-4. What arethe equilibrium concentrations of Ca2 + and CrO42- in aa saturated solution of CaCrO4?A certain reaction has an equilibrium constant of 10.1 at 25oC. A ⇄ B Determine its ΔG, in kJ/mol, if [A]=0.05 M and [B]=1x10-4 M?

