Which of the following acid/ conjugate base pairs would function best as a buffer at physiological pH? Lactic acid / Lactate ion; pKa = 3.86 Carbonic acid / Bicarbonate ion; pka = 6.37 Bicarbonate ion / Carbonate ion; pka = 10.25 Dihydrogen phosphate / Monohydrogen phosphate ion; pKa = 6.86 Acetic acid / Acetate ion; pKa = 4.76
Which of the following acid/ conjugate base pairs would function best as a buffer at physiological pH? Lactic acid / Lactate ion; pKa = 3.86 Carbonic acid / Bicarbonate ion; pka = 6.37 Bicarbonate ion / Carbonate ion; pka = 10.25 Dihydrogen phosphate / Monohydrogen phosphate ion; pKa = 6.86 Acetic acid / Acetate ion; pKa = 4.76
Introduction to General, Organic and Biochemistry
11th Edition
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Chapter31: Immunochemistry
Section: Chapter Questions
Problem 31.72P
Related questions
Question
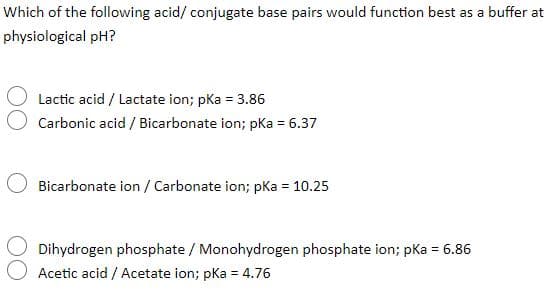
Transcribed Image Text:Which of the following acid/ conjugate base pairs would function best as a buffer at
physiological pH?
Lactic acid / Lactate ion; pka = 3.86
Carbonic acid / Bicarbonate ion; pka = 6.37
Bicarbonate ion / Carbonate ion; pKa = 10.25
Dihydrogen phosphate / Monohydrogen phosphate ion; pKa = 6.86
Acetic acid / Acetate ion; pka = 4.76
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps

Recommended textbooks for you

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning