Which of the following applies to the following pair of molecules? VS. Select one: A. They are resonance structures. O B. They are identical compounds. O C. They are constitutional isomers. D. They are different compounds, and not isomers.
Which of the following applies to the following pair of molecules? VS. Select one: A. They are resonance structures. O B. They are identical compounds. O C. They are constitutional isomers. D. They are different compounds, and not isomers.
Chapter4: Forces Between Particles
Section: Chapter Questions
Problem 4.77E
Related questions
Question
HW10
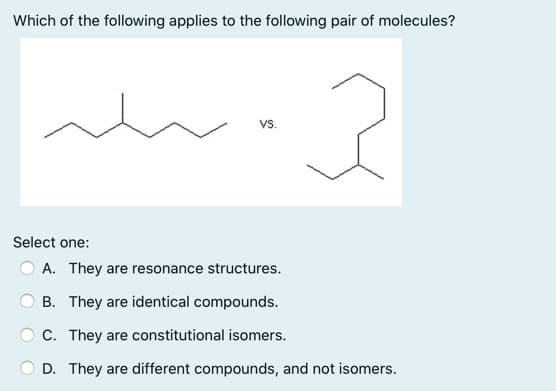
Transcribed Image Text:Which of the following applies to the following pair of molecules?
Vs.
Select one:
A. They are resonance structures.
B. They are identical compounds.
C. They are constitutional isomers.
D. They are different compounds, and not isomers.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 1 images

Recommended textbooks for you


Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:
9781559539418
Author:
Angelica Stacy
Publisher:
MAC HIGHER


Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:
9781559539418
Author:
Angelica Stacy
Publisher:
MAC HIGHER