Which of the following best describes the reaction of Cu(s) +HNO3(ag)→? Select all that apply. Copper dissolved into a blue solution No fumes were formed Heat was formed (exothermic) Copper dissolved into a green solution Copper did not dissolve White fumes were formed Heat was consumed (endothermic) Brown fumes were formed Submit Previous Answers Request Answer P Pearso Copyright © 2020 Pearson Education Inc. All rights reserved. Te DELL XLI
Which of the following best describes the reaction of Cu(s) +HNO3(ag)→? Select all that apply. Copper dissolved into a blue solution No fumes were formed Heat was formed (exothermic) Copper dissolved into a green solution Copper did not dissolve White fumes were formed Heat was consumed (endothermic) Brown fumes were formed Submit Previous Answers Request Answer P Pearso Copyright © 2020 Pearson Education Inc. All rights reserved. Te DELL XLI
Chapter18: Electrochemistry
Section: Chapter Questions
Problem 85E: Under standard conditions, what reaction occurs, if any, when each of the following operations is...
Related questions
Question
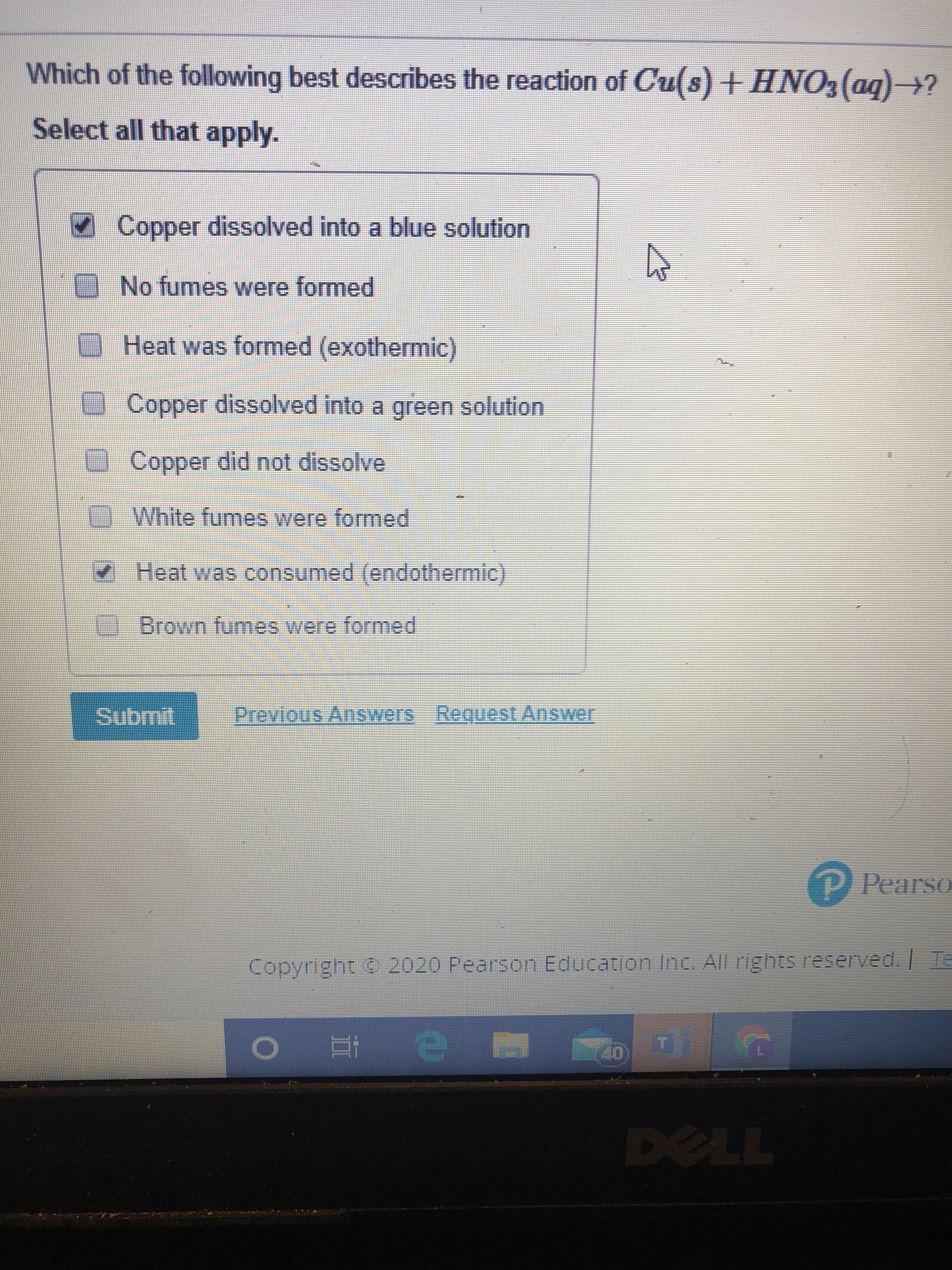
Transcribed Image Text:Which of the following best describes the reaction of Cu(s) +HNO3(ag)→?
Select all that apply.
Copper dissolved into a blue solution
No fumes were formed
Heat was formed (exothermic)
Copper dissolved into a green solution
Copper did not dissolve
White fumes were formed
Heat was consumed (endothermic)
Brown fumes were formed
Submit
Previous Answers Request Answer
P Pearso
Copyright © 2020 Pearson Education Inc. All rights reserved. Te
DELL
XLI
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Recommended textbooks for you


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning