Which of the following does not describe the molecule shown? ОН Figure 12 It could form an ionic bond with a molecule carrying a negative charge. e. It would easily dissolve in water. It is capable of forming a hydrogen bond with the oxygen atom on a water molecule. It could form an ionic bond with a molecule carrying a positive charge. It is capable of forming a hydrogen bond with a hydrogen atom on a water molecule.
Which of the following does not describe the molecule shown? ОН Figure 12 It could form an ionic bond with a molecule carrying a negative charge. e. It would easily dissolve in water. It is capable of forming a hydrogen bond with the oxygen atom on a water molecule. It could form an ionic bond with a molecule carrying a positive charge. It is capable of forming a hydrogen bond with a hydrogen atom on a water molecule.
Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter2: Atoms, Molecules, And Ions
Section: Chapter Questions
Problem 105AE
Related questions
Question
do question 5
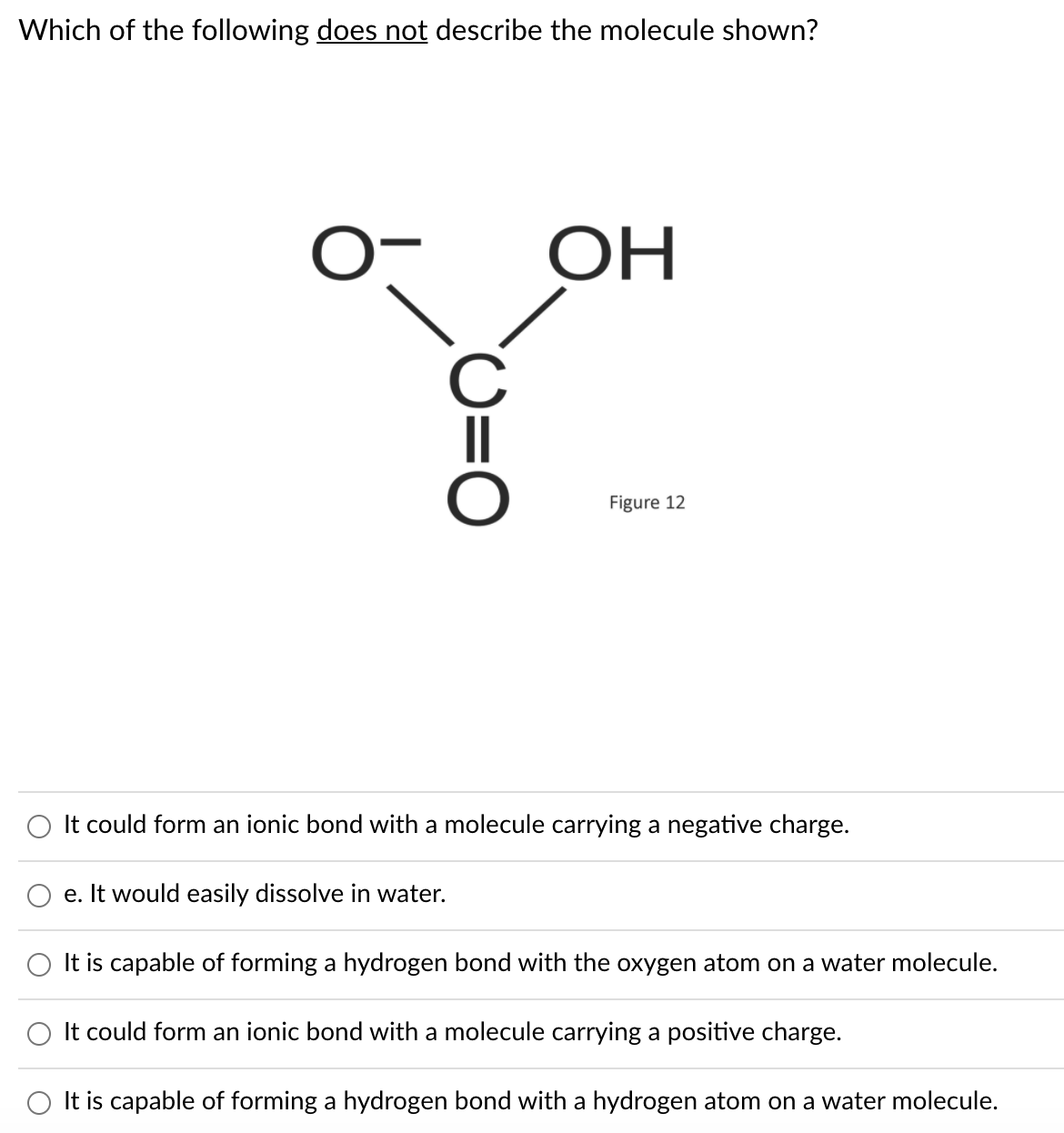
Transcribed Image Text:Which of the following does not describe the molecule shown?
OH
C
Figure 12
O It could form an ionic bond with a molecule carrying a negative charge.
e. It would easily dissolve in water.
It is capable of forming a hydrogen bond with the oxygen atom on a water molecule.
It could form an ionic bond with a molecule carrying a positive charge.
O It is capable of forming a hydrogen bond with a hydrogen atom on a water molecule.
Expert Solution
Step 1
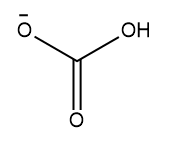
The given species is an anion, and it contains a negative charge on the oxygen atom. The structure of the given species is as follows:
Trending now
This is a popular solution!
Step by step
Solved in 4 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning


Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co