Which of the following is correct? Select one: Oa. 75.0 cm Hg = 75.0 torr %3D Ob. 153 kPa = 1.55 x 10 atm. O C. 25.0 psi =172 kPa %3D O d. 5.64 x 10 atm = 742 mm Hg O e. 5.99 x 10 in. Hg 1.77 x 10 Pa
Which of the following is correct? Select one: Oa. 75.0 cm Hg = 75.0 torr %3D Ob. 153 kPa = 1.55 x 10 atm. O C. 25.0 psi =172 kPa %3D O d. 5.64 x 10 atm = 742 mm Hg O e. 5.99 x 10 in. Hg 1.77 x 10 Pa
Chemistry & Chemical Reactivity
9th Edition
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter10: Gases And Their Properties
Section10.2: Gas Laws: The Experimental Basis
Problem 1RC
Related questions
Question
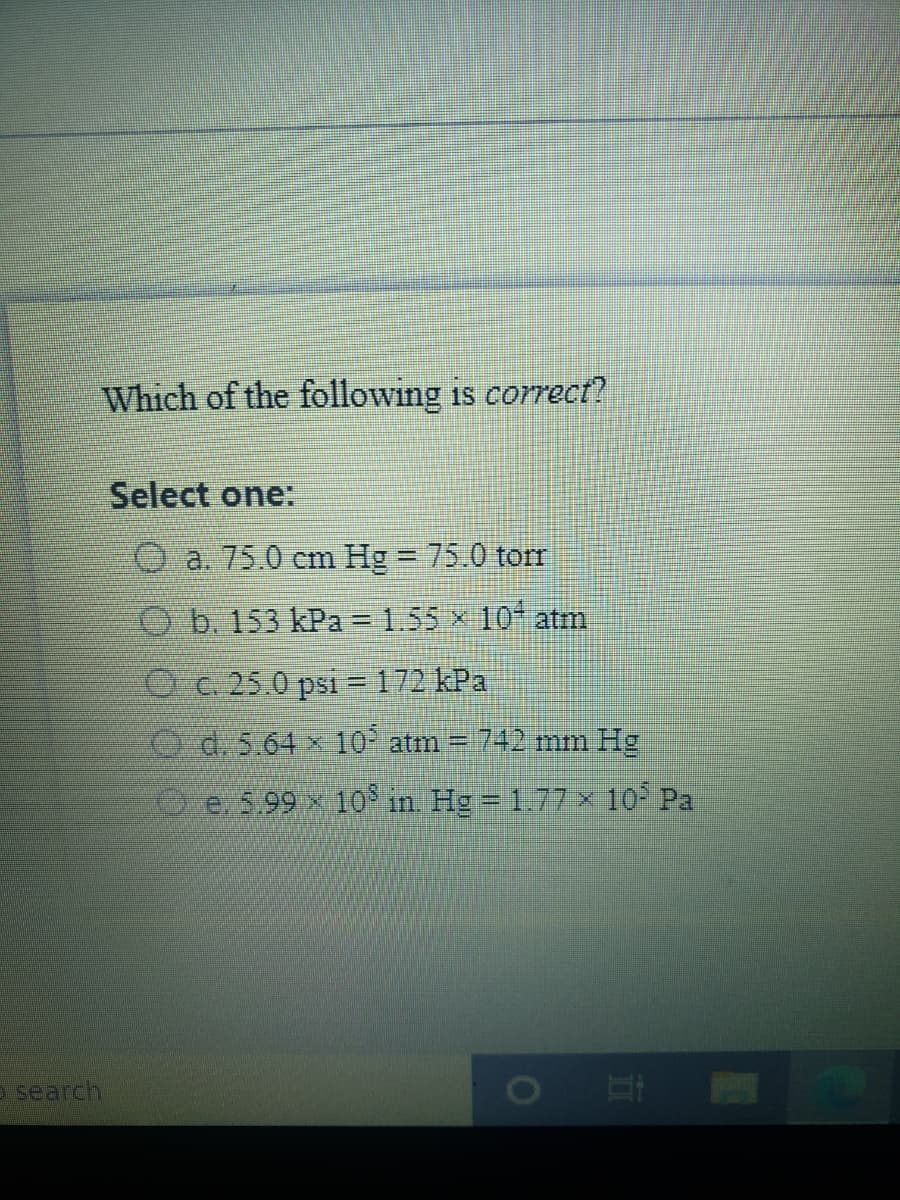
Transcribed Image Text:Which of the following is correct?
Select one:
a. 75.0 cm Hg = 75.0 torr
O b. 153 kPa = 1.55 x 10- atm
Oc. 25.0 psi =172 kPa
O d. 5.64 x 10° atm = 742 mm Hg
O e. 5.99 x 10 in. Hg = 1.77 × 10 Pa
- search
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning