Which of the following is not true about low molar mass carboxylic acids? They have a sour taste. They change red litmus to blue. They form aqueous solutions with a pH less than 7. They react with bases to form water and a salt. Which of the following statements best describes the solubility of aliphatic carboxylic acids in water? The solubility decreases as the number of carbon atoms increase. The fist 4-carbon acids are miscible with water, while those with 8 or more carbons are insoluble. The solubility increases as the number of carbon atoms decrease. All of them are soluble in water. What is the order of increasing acidity for the following compounds? (least to most) COOH COOH COOH COOH CH3 NO₂ I II III IV IV, I, III, II III, II, I, IV II, III, I, IV III, II, IV, I
Which of the following is not true about low molar mass carboxylic acids? They have a sour taste. They change red litmus to blue. They form aqueous solutions with a pH less than 7. They react with bases to form water and a salt. Which of the following statements best describes the solubility of aliphatic carboxylic acids in water? The solubility decreases as the number of carbon atoms increase. The fist 4-carbon acids are miscible with water, while those with 8 or more carbons are insoluble. The solubility increases as the number of carbon atoms decrease. All of them are soluble in water. What is the order of increasing acidity for the following compounds? (least to most) COOH COOH COOH COOH CH3 NO₂ I II III IV IV, I, III, II III, II, I, IV II, III, I, IV III, II, IV, I
Introductory Chemistry: A Foundation
9th Edition
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Donald J. DeCoste
Chapter20: Organic Chemistry
Section: Chapter Questions
Problem 146CP
Related questions
Question
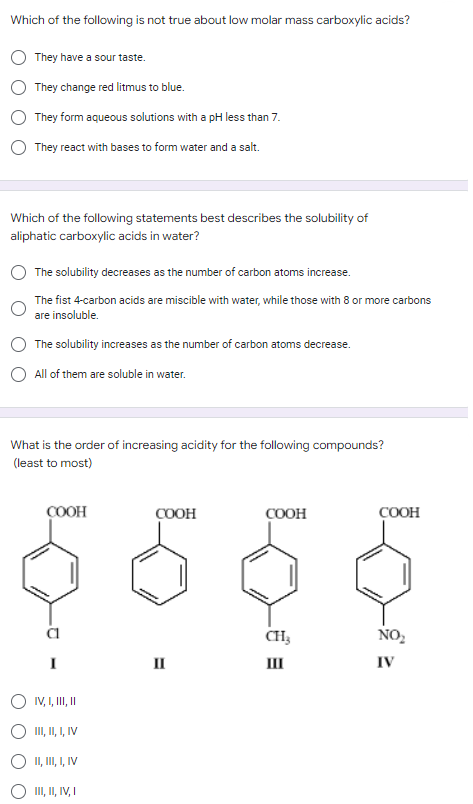
Transcribed Image Text:Which of the following is not true about low molar mass carboxylic acids?
They have a sour taste.
They change red litmus to blue.
They form aqueous solutions with a pH less than 7.
They react with bases to form water and a salt.
Which of the following statements best describes the solubility of
aliphatic carboxylic acids in water?
The solubility decreases as the number of carbon atoms increase.
The fist 4-carbon acids are miscible with water, while those with 8 or more carbons
are insoluble.
The solubility increases as the number of carbon atoms decrease.
All of them are soluble in water.
What is the order of increasing acidity for the following compounds?
(least to most)
COOH
COOH
COOH
COOH
CH3
NO₂
I
III
IV
IV, I, III, II
III, II, I, IV
II, III, I, IV
III, II, IV, I
II
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Organic And Biological Chemistry
Chemistry
ISBN:
9781305081079
Author:
STOKER, H. Stephen (howard Stephen)
Publisher:
Cengage Learning,

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Organic And Biological Chemistry
Chemistry
ISBN:
9781305081079
Author:
STOKER, H. Stephen (howard Stephen)
Publisher:
Cengage Learning,

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning