Which of the following molecules cannot exhibit intermolecular (among themselves) hydrogen bonding? O ammonia hydrogen sulfide O flourine gas O ethanol O butanol
Which of the following molecules cannot exhibit intermolecular (among themselves) hydrogen bonding? O ammonia hydrogen sulfide O flourine gas O ethanol O butanol
Chemistry: An Atoms First Approach
2nd Edition
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Steven S. Zumdahl, Susan A. Zumdahl
Chapter14: Acid- Base Equilibria
Section: Chapter Questions
Problem 4RQ: A good buffer generally contains relatively equal concentrations of weak acid and conjugate base. If...
Related questions
Question
Question 7
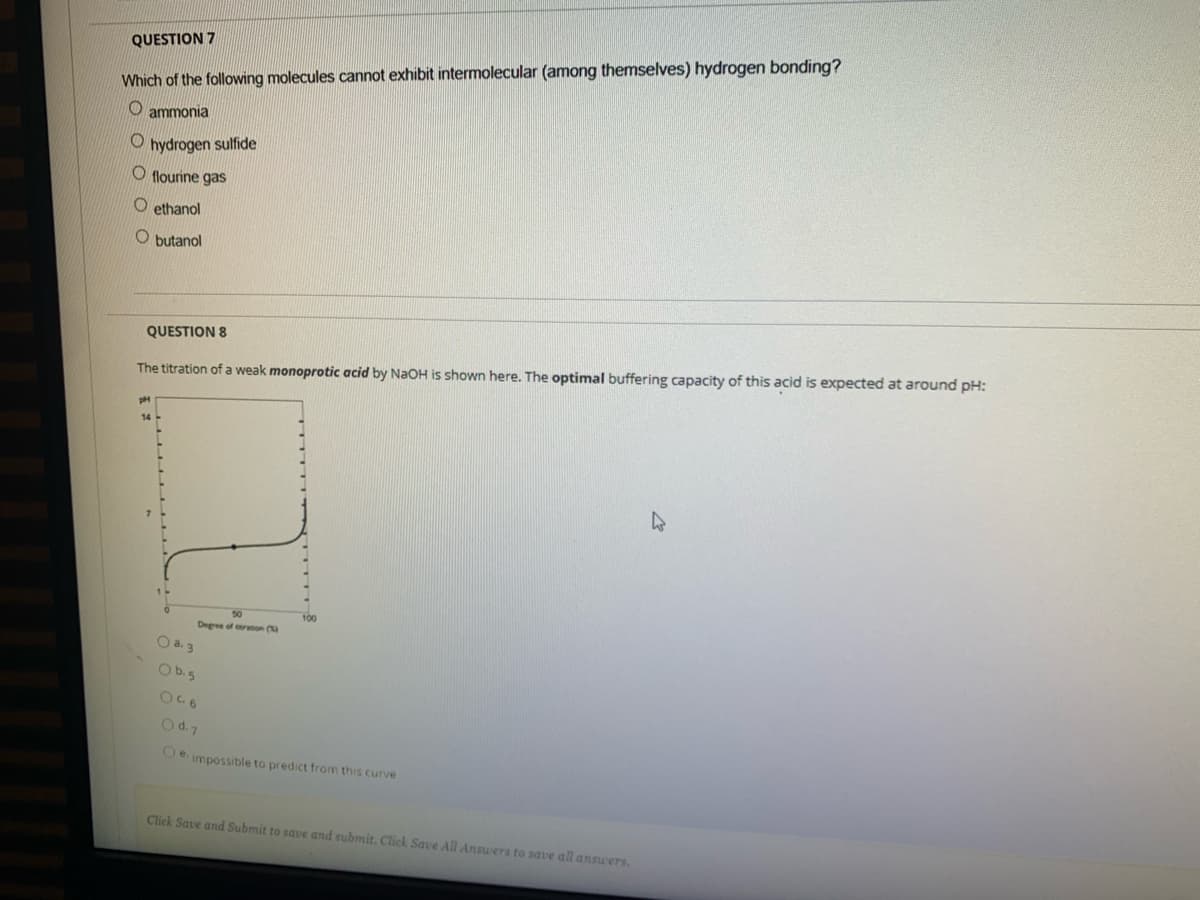
Transcribed Image Text:QUESTION 7
Which of the following molecules cannot exhibit intermolecular (among themselves) hydrogen bonding?
O ammonia
hydrogen sulfide
O flourine gas
O ethanol
O butanol
QUESTION 8
The titration of a weak monoprotic acid by NaOH is shown here. The optimal buffering capacity of this acid is expected at around pH:
50
100
Degree of ron (
O a. 3
Ob.5
Od.7
Oe. impossible to predict from this curve
Click Save and Submit to save and submit. Click Save All Answers to save all answers.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning
