Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter20: Chemistry Of The Metals
Section: Chapter Questions
Problem 34QAP: The equilibrium constant for the reaction 2CrO42(aq)+2H+(aq)Cr2O72(aq)+H2O is 31014 . What must the...
Related questions
Question
Transcribed Image Text:10:29 PM Mon Oct 18
94%
A adamson.blackboard.com
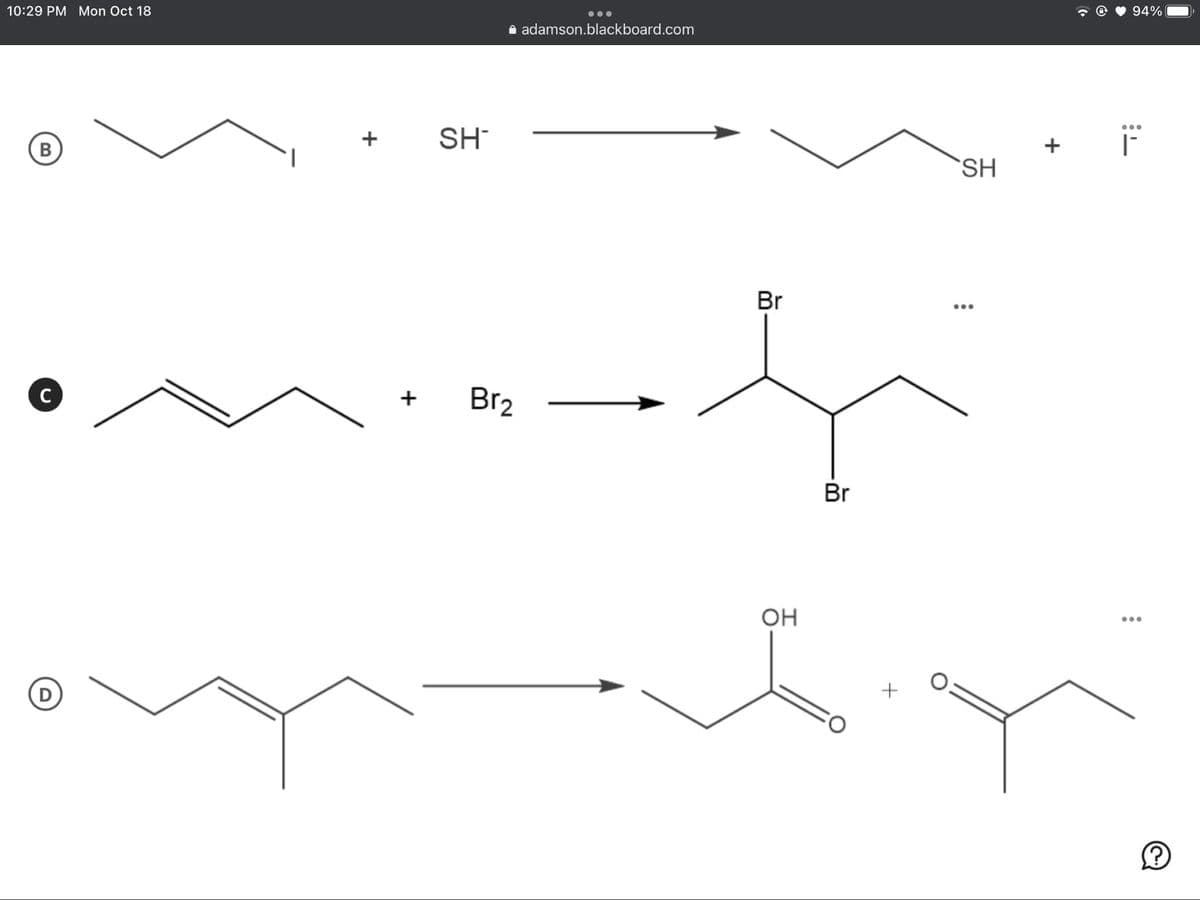
SH-
В
SH
Br
...
Br2
C
+
Br
Он
+
+
Transcribed Image Text:10:29 PM Mon Oct 18
94%
adamson.blackboard.com
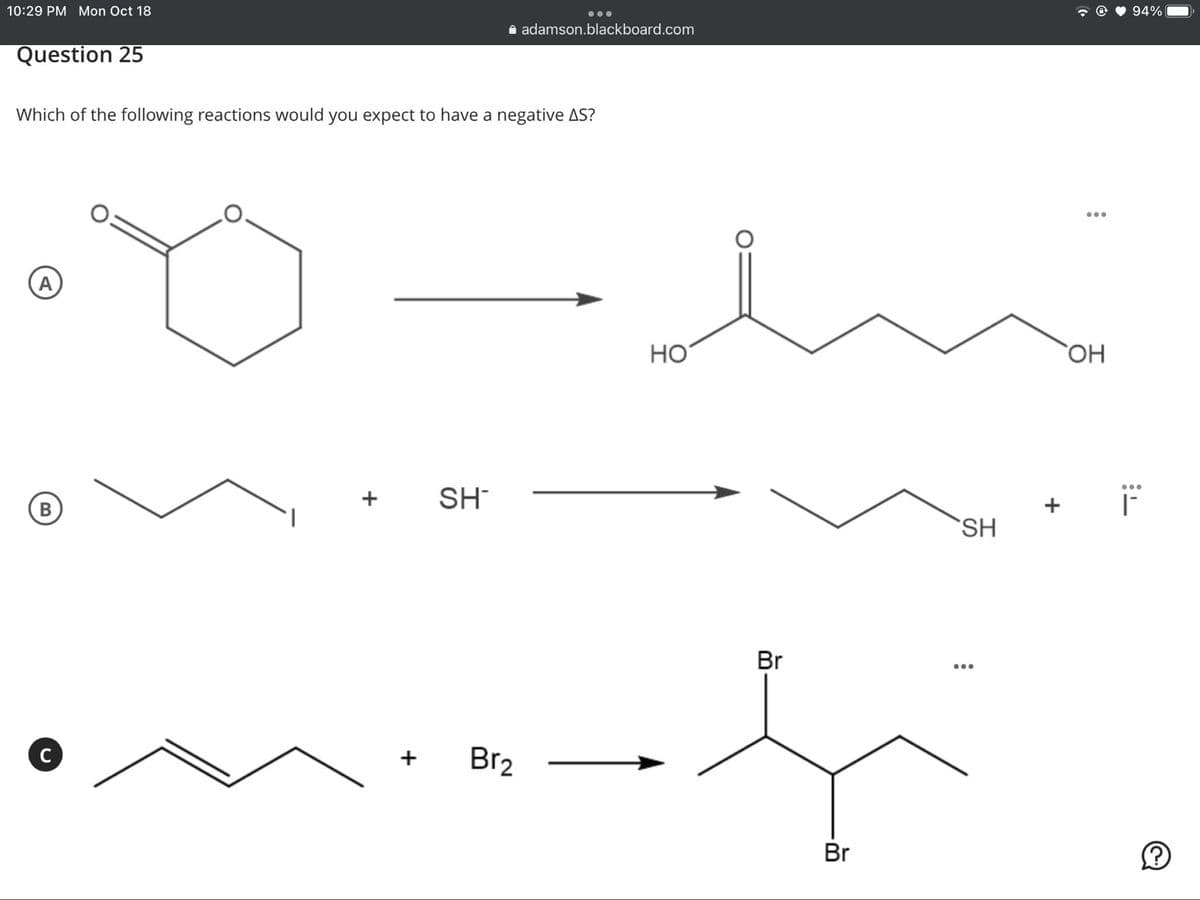
Question 25
Which of the following reactions would you expect to have a negative AS?
...
A
Но
...
SH
B
В
+
SH
Br
...
Br2
Br
+
+
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning