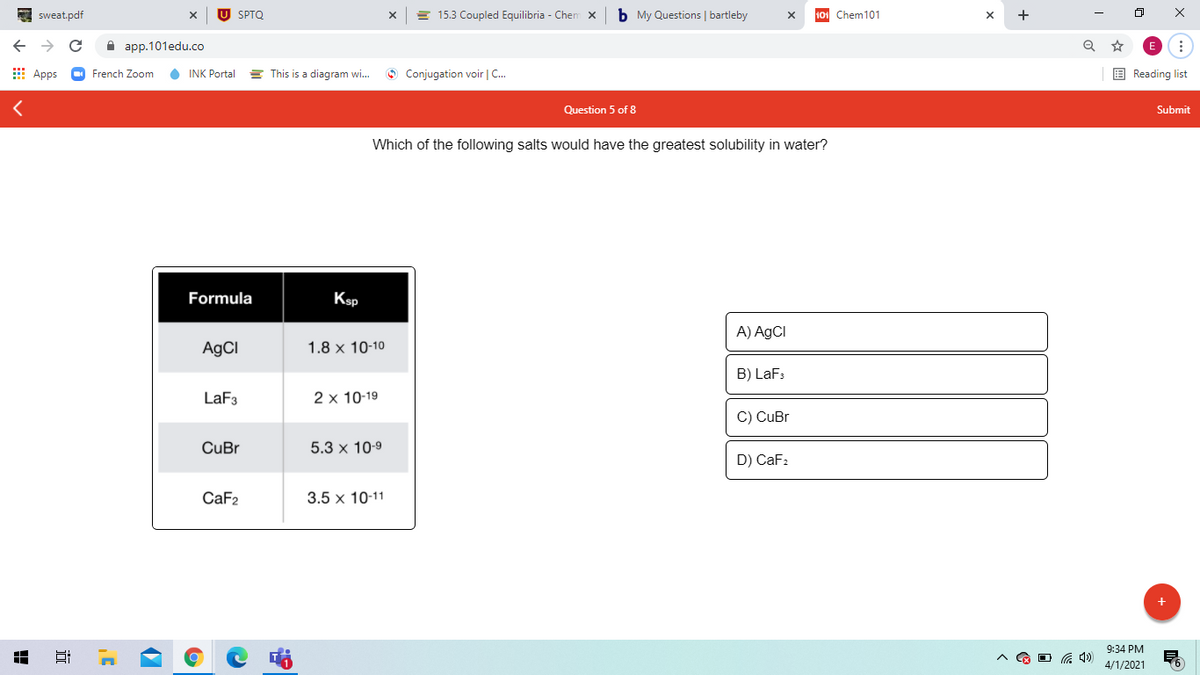
Which of the following salts would have the greatest solubility in water? Formula Ksp A) AGCI AgCI 1.8 x 10-10 B) LaFs LaF3 2 x 10-19 C) CuBr CuBr 5.3 x 10-9 D) CaF: CaF2 3.5 x 10-11
Q: Which salt below has the greatest molar solubility in water? PbCrO4 (Ksp = 1.8 x 10-14) Cul (Ksp =…
A: Molar solubility: Molar solubility in water is the number of moles of solute that is soluble in one…
Q: Q2- How mainy mls of Atropine sulphate present in lab. As a stock sol. Of 1: 20 conc. should be used…
A: 1:20 concentration indicate that 20 mL of stock solution contains 1 g of atropine sulphate.
Q: What is the [OH¯] of a 3.50 M solution of pyridine (C5H5N, Kb = 1.70 × 10-9)?
A: Pyridine is a weak base so in solution the OH- concentration of pyridine will be very much less. It…
Q: a soultion has a concentration of 6.00 and contains 1.45g of HNO3. What is the voulume of this…
A: Given Concentration = 6 mol/L mass = 1.45 g
Q: What is the percent ionization of a 0.25 M HC2H3O2 solution (Ka = 1.8 x 10-5) at 25°C? O 0.60 % 0.42…
A: We have to find percent ionisation
Q: 7. How many mL of a 0.20 M NAOH solution are needed to neutralize 10 mL of 0.30 M H2SO4 solution.…
A: The given reaction is : 2 NaOH + H2SO4 → Na2SO4 + 2H2O Molarity of NaOH (M1) = 0.20 M Volume of NaOH…
Q: What volume of 0.150 M NaOH is needed to neutralize 35.00mL of 0.250 M H2S
A: Given data : For NaOH --> Molarity(M1) = 0.15M Volume(V1) =? For H2S ----> Molarity(M2) =…
Q: What is the approximate freezing point of a 0.50 M solution of dichloroacetic acid, HC2HO2Cl2…
A:
Q: ► Get the concentration of the unknown solutions with %T of 63. ►Given: S1-0.02 mg 52-0.04 mg…
A: Here we are required to find the concentration of unknown solution with transmittance (%T )63…
Q: a. Atropine sulphate 1.2% Boric acid q.s. Purified water q.s. ad 15ml M. ft. ophth. sol. Calculate…
A: We’ll answer the first question since the exact one wasn’t specified. Please submit a new question…
Q: How much water must you add to 25 mL of 3.0 M NH3 to dilute it to 0.60M?
A: Since we are just adding water Hence moles of NH3 in initial solution should be equal to moles of…
Q: What is the ppm concentration of 6.00mL of solution that has 3.6×10-4g of soduim ions
A: Welcome to bartleby !
Q: How many grams of Amilin çHs NHz Should be Solved in water to Prefare I liter containg oH iong Know…
A: Let the initial concentration of aniline be A. The ICE table for the reaction is given below :…
Q: How many milliliters of 0.50 M NaOH solution are required to titrate 40.0 mL of a 0.10 M H2SO4…
A: Introduction: Molarity: Molarity is defined as the number of moles of solute in per litre of…
Q: Solution 1: How would you prepare a 250 mL solution of .245 M H3PO4? Solution 2: How would you…
A: The molarity of a solution defines the concentration of that solution. It is given as the number of…
Q: Prepare 100 ml each of 0.1 M HCL and 0.1 M NaOH.
A: Molarity is a way of expressing concentration in which moles of solute present per liter of solution…
Q: . Which of the following combinations will produce a0.4 M NaCl solution?(A) Mixing 500 mL of 0.4 M…
A: Given that 0.4 M NaCl has to be produced. Number of moles can be calculated from the Molarity:
Q: What is the percent ionization of a 0.50 M HC2H3O2 solution (Ka = 1.8 x 10-5) at 25°C? O 0.15 % 0.38…
A:
Q: Which monovalent hydroxide below supports the following data: A 0.515 g sample dissolved in 24.7…
A:
Q: A solution is composed of 30 mL of 2.5 M H2SO4 and 25 mL of 6.4 N KOH. What is the milliequivalences…
A: Given, Concentration of H2SO4 is ----- 2.5M Volume of H2SO4 is -----30mL. How many milliequivalents…
Q: Which of the following is the least soluble in water? Select one: O a. NaCl (sodium chloride) O b.…
A: The NaCl which is commonly known as sodium chloride is strongly soluble in water on dissolving and…
Q: What is the percent ionization of a 0.10 M HF solution (Ka = 6.8*10-4)? 0.082% 0.82% 0.0082% 8.2%…
A: Given Details: - Ka = 6.8 x 10-4 Value of HF solution = 0.10 M To find: Percent…
Q: Calculate the molarity of a solution when 10.0g NaOH (MM=40.00g/mol) are dissolved. a. 0.0667m…
A: so, the Molarity of NaOH per litter = 10g/(40g/mole) = 0.25M So it is near answer is 0.267m
Q: 25.0 mL
A: Please find the attached file for explanation
Q: Choose the letter that ranks the numbers from Least Soluble to Most Soluble when mixed with H,O.…
A: Ans The solubility of the solute in the water decide by the hydrogen bonding of the molecules…
Q: Chap Explain how you would make the following solutions. 10) 2 L of 3 M HCI
A: Answer
Q: Indicate the solubility in 5% HCl and solubility class of the following compounds. CI insol. -S…
A:
Q: 7- How would you classify KCIO4? a) acidic salt b) alkaline salt c) neutral salt d) a weak salt
A: KClO4 is obtained by neutralization reaction of KOH and HClO4.
Q: What is the percent ionization of a 0.50 M HC2H3O2 solution (Ka = 1.8 x 10-5 ) at 25°C? O 0.15 %…
A: Given: Concentration of HC2H3O2 = 0.50 M And Ka of HC2H3O2 = 1.8 X 10-5 Since HC2H3O2 is a weak…
Q: How many mL of 2.50 M H2SO4 are required to neutralize 30.0 mL of 1.25 M NaOH? balanced equation:…
A: Whenver an acid and base reacts with each other than they form salt and water and the process is…
Q: Prepare 100 ml each of 0.1 M HCL and 0.1 M NaOH
A: Molarity is a way of expressing concentration which indicates the moles of solute present per liter…
Q: If your drug is a weak base then to increase its solubility in water you should: Select one: O a.…
A:
Q: If 1 CN- ion kills 10 cancer cells, how many cells would be killed by drinking 25 ml of a saturated…
A: Cu(CN)2 <--------> Cu+2 + 2CN-
Q: Q2- How many mls of Atropine sulphate present in lab. As a stock sol. Of 1: 20 conc. should be used…
A: A question based on concentration terms that is to be accomplished.
Q: 8. Which of these two would you expect to be more soluble in water? Why? benzoic acid butanoic acid…
A:
Q: 7- How much Kl is required to prepare 100 mL of 0.25M KI solution?
A: * molarity = Number of moles / volume of…
Q: NH vesulting from wH- Cl needed to provent mgl oH)2 From precipitating in alite of a S olution…
A:
Q: (a) Read the following burette and graduated cylinder and record your answer in the spaces provided…
A: The ratio of the given mass to the molar mass gives the number of moles.
Q: Compared to pure water, a 1 M sugar-water solution will have a? A. lower VP, lower BP, and lower FP…
A: The correct option is (D) It is because sugar is non- volatile solute which added to water i.e…
Q: 13 which Of these aqueous SOIutions conduct eiectricity well? O acetone, CH3 COCH3 D AGNO3 caci2…
A: Which of the aqueous solution conduct electricity well and why they conduct electricity explanation…
Q: If lg of drug A replaces 0.5g of cocoa butter and lg of drug B replaces 0.25g of cocoa butter then…
A: 1g drug displace 0.5g cocoa butter. Then density factor=wt Of drug/wt of base displaced. =1g/0.5 g…
Q: One liter of a 1M NaCl solution has less Na molecules than K molecules in one liter of a 1M KCI…
A: The statement is False. The sodium is smaller in size than potassium atom because from periodic…
Q: What is the percent ionization of a 0.50 M HC2H3O, solution (Ka = 1.8 × 10-5 ) at 25°C? 0.42 % 0.15…
A: Given: Concentration of HC2H3O2 = 0.50 M And Ka of HC2H3O2 = 1.8 X 10-5 Since HC2H3O2 is a weak…
Q: what will the concentration be if 500.0 ml of water are aded to 75.0 ml of 3.0 M HNO3?
A: The case is of a dilution reaction. Given : M1 = 3.0 M V1 = 57 mL 500 mL of water is…
Q: Calculate the pHpH of each solution 1. [H3O+] = 3.7×10−8 M 2. [H3O+] = 6.0×10−7 M 3.[H3O+] =…
A: The question have given the concentration of the hydronium ion and we have to calculate the PH of…
Q: What volume of 0.25 M HCl must be diluted to prepare 1.4 L of 7.0×10-2M HCl? _________ L
A: Molarity of a solution is used to express the concentration of the unknown solution. It can be…
Q: What is the solubility of nitrogen gas at 25*C
A: The given graph for the question is :
Q: 15) How many milliliters of 0,225M HaPO, WOuld be needed to react exactly with 20.0mL of 0.135M…
A:
Q: The [OH'] x [H*] in neutral water at 25°C is 10-14 True False
A:
Which of the following salts would have the greatest solubility in water?
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

- The Henry’s law constant for CO2 in water at 25 °C is3.1x 10-2 M atm-1. (a) What is the solubility of CO2 inwater at this temperature if the solution is in contact withair at normal atmospheric pressure? (b) Assume that all ofthis CO2 is in the form of H2CO3 produced by the reactionbetween CO2 and H2O:CO2(aq) + H2O(l)------>H2CO3(aq)What is the pH of this solution?A solution is prepared by dissolving 40.00 g of NaCl (f.w. = 58.44 g mol–1), a non-volatile solute, in enough water (m.w. = 18.02 g mol–1) to result in exactly 1 L of solution at 25 °C. Assume the density of the solution is that of pure water (dsolution = 1.000 g mL–1). The ebullioscopic constant (Kb) for water is 0.513 °C m–1. The cryoscopic constant (Kf) for water is 1.86 °C m–1. The vapor pressure of pure water is 0.0313 atm. Find the freezing point of the solution(in C to 2 decimal places)A solution is prepared by dissolving 40.00 g of NaCl (f.w. = 58.44 g mol–1), a non-volatile solute, in enough water (m.w. = 18.02 g mol–1) to result in exactly 1 L of solution at 25 °C. Assume the density of the solution is that of pure water (dsolution = 1.000 g mL–1). The ebullioscopic constant (Kb) for water is 0.513 °C m–1. The cryoscopic constant (Kf) for water is 1.86 °C m–1. The vapor pressure of pure water is 0.0313 atm. Find the osmotic pressure in atm to three decimal places
- A solution is prepared by dissolving 40.00 g of NaCl (f.w. = 58.44 g mol–1), a non-volatile solute, in enough water (m.w. = 18.02 g mol–1) to result in exactly 1 L of solution at 25 °C. Assume the density of the solution is that of pure water (dsolution = 1.000 g mL–1). The ebullioscopic constant (Kb) for water is 0.513 °C m–1. The cryoscopic constant (Kf) for water is 1.86 °C m–1. The vapor pressure of pure water is 0.0313 atm. Determine the boiling point of the solution(in C to 2 decimal places)A solution is prepared by dissolving 40.00 g of NaCl (f.w. = 58.44 g mol–1), a non-volatile solute, in enough water (m.w. = 18.02 g mol–1) to result in exactly 1 L of solution at 25 °C. Assume the density of the solution is that of pure water (dsolution = 1.000 g mL–1). The ebullioscopic constant (Kb) for water is 0.513 °C m–1. The cryoscopic constant (Kf) for water is 1.86 °C m–1. The vapor pressure of pure water is 0.0313 atm. Determine the following: Boiling point of solution (in °C to two decimal places) Freezing point of solution (in °C to two decimal places) Vapor pressure of the solution (in atm to three decimal places) Osmotic pressure (in atm to three decimal places)What is the boiling point rise in oF of a 15% solution of nitric acid in water boiling at 200oF? Need asap
- 1. Calculate the solubility of calcium carbonate water temperature of 5 °C; at that temperature, Ksp = 8.1 x 10-9 for CaCO3, Ka = 2.8 x 10-11 for HCO3-, and Kw = 2.0 x 10-15An aqueous chloric acid solution has a sp. gr. of 1.124 and contains 26.02% by mass. Calculate M, N, m, mole fraction of solute, BP, FP, and osmotic pressure at 25.0°C assuming 100% effective ionization.KNO3 (aq) added to CaC2O4 (Ksp = 1.3 x 10-8) in solution will lead to __________ in solubility of the solid no effect then decrease increase no effect
- 23. The Ksp of CuCO3ls) (copper carbonate, a salt) is reported as 2.21x10-11. Determine the solubility (in mg/L) of 1this salt in pure water Constants: The molecular mass (M) of copper carbonate (CuCOg) is 123.554 g mol-12.3 - At temperature of 18°C was 4,7 g of silver molybdate (M = 376 g mol–1) mixed with 500 cm3 of a) distilled water b) solution of AgNO3 with concentration of 0,02 mol dm–3 c) solution of Na2MoO4 with concentration of 0,02 mol dm–3 Determine, how many percent of Ag2MoO4 will dissolve in each case. Solubility equilibrium of Ag2MoO4 is 3,1.10-11.A solution is prepared by dissolving 40.00 g of MgCl2 (f.w. = 95.211 g mol–1), a non-volatile solute, in enough water (m.w. = 18.02 g mol–1) to result in exactly 2 L of solution at 25 °C. Assume the density of the solution is that of pure water (dsolution = 1.000 g mL–1). The ebullioscopic constant (Kb) for water is 0.513 °C m–1. The cryoscopic constant (Kf) for water is 1.86 °C m–1. The vapor pressure of pure water is 0.0313 atm. Determine the freezing point of the solution.

