Which of the following statements is TRUE? 1. Fe2+ form a 1:6 complex with 1,10-phenanthroline II. The iron (II)-phenanthroline complex displays an intense orange-colored solution. III. The formation constant of Fe²+ is higher than Fe³+ with 1,10-phenanthroline complexing agent. IV. 1,10-phenanthroline is a heterocyclic compound containing three nitrogen atoms. A) I and III B) II and III I and II D) II and IV only
Which of the following statements is TRUE? 1. Fe2+ form a 1:6 complex with 1,10-phenanthroline II. The iron (II)-phenanthroline complex displays an intense orange-colored solution. III. The formation constant of Fe²+ is higher than Fe³+ with 1,10-phenanthroline complexing agent. IV. 1,10-phenanthroline is a heterocyclic compound containing three nitrogen atoms. A) I and III B) II and III I and II D) II and IV only
Chapter17: Complexation And Precipitation Reactions And Titrations
Section: Chapter Questions
Problem 17.11QAP
Related questions
Question
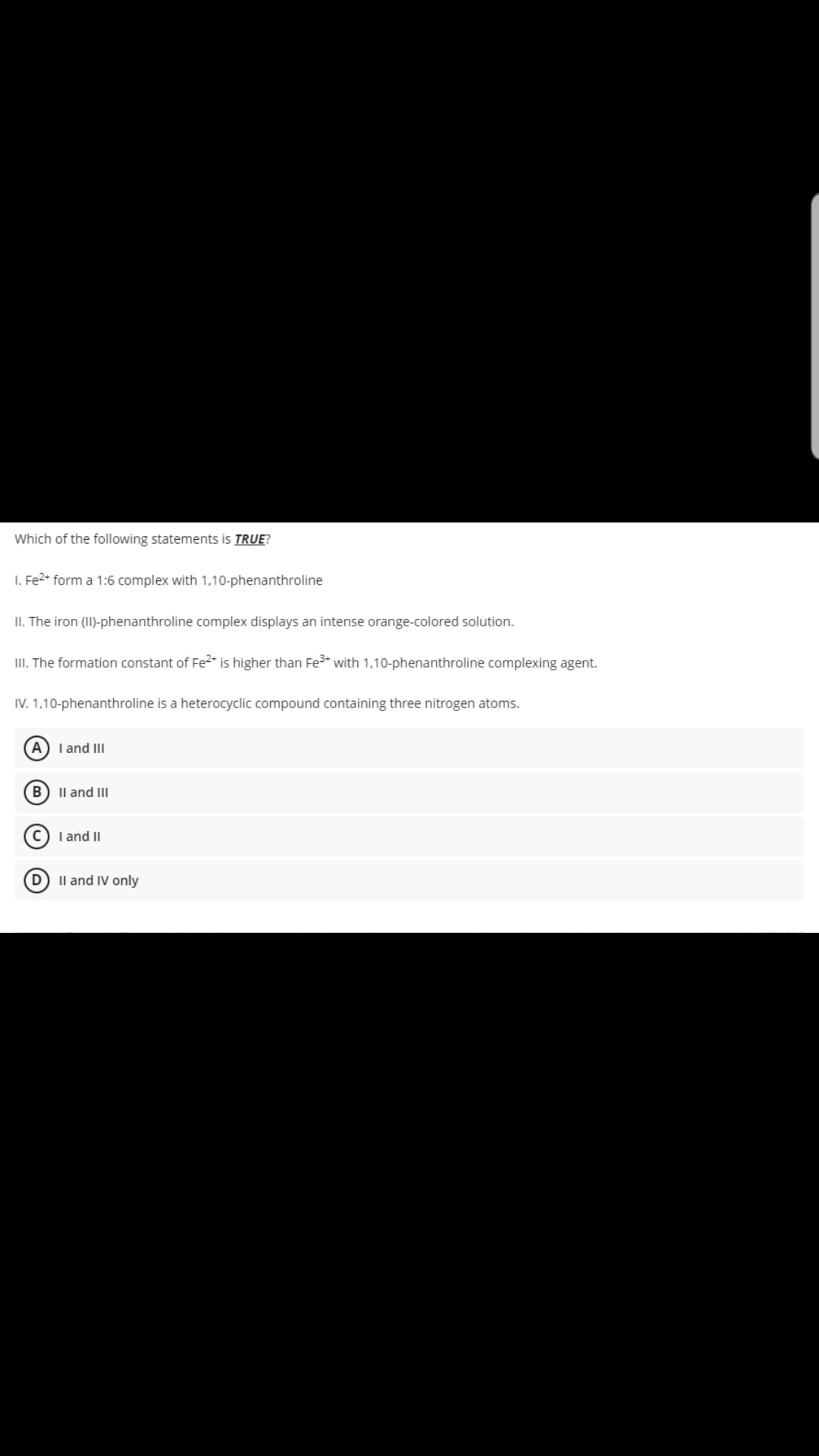
Transcribed Image Text:Which of the following statements is TRUE?
1. Fe2+ form a 1:6 complex with 1,10-phenanthroline
II. The iron (II)-phenanthroline complex displays an intense orange-colored solution.
III. The formation constant of Fe2+ is higher than Fe³+ with 0-phenant
IV. 1,10-phenanthroline is a heterocyclic compound containing three nitrogen atoms.
(A) I and III
B II and III
C) I and II
II and IV only
complexing agent.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning


Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning