Which of the following will cause no change in the amount of Fe calculated from the results of an iron-phenanthroline analysis? (A) No added hydroxylamine B All of the given choices will cause a change in the calculated Fe content of the sample Using a pH 10 buffer D Using 0.5% w/v 1,10-phenanthroline instead of 0.1% w/v.
Which of the following will cause no change in the amount of Fe calculated from the results of an iron-phenanthroline analysis? (A) No added hydroxylamine B All of the given choices will cause a change in the calculated Fe content of the sample Using a pH 10 buffer D Using 0.5% w/v 1,10-phenanthroline instead of 0.1% w/v.
Chapter17: Complexation And Precipitation Reactions And Titrations
Section: Chapter Questions
Problem 17.33QAP
Related questions
Question
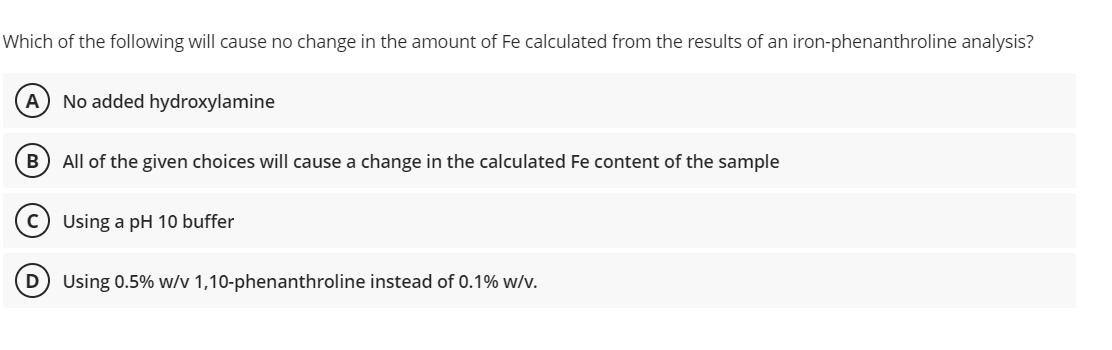
Transcribed Image Text:Which of the following will cause no change in the amount of Fe calculated from the results of an iron-phenanthroline analysis?
A No added hydroxylamine
B) All of the given choices will cause a change in the calculated Fe content of the sample
C) Using a pH 10 buffer
Using 0.5% w/v 1,10-phenanthroline instead of 0.1% w/v.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 5 steps with 4 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you



Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning



Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning