Which pair of elements have the same number of valence electrons? O Do and Wh (elements 119 and 137 on special periodic table) O Wf and Bd (elements 148 and 146 on special periodic table) O Hr and Ba (elements 120 and 56 on special periodic table) O Wh and Rb (elements 137 and 37 on special periodic table)
Which pair of elements have the same number of valence electrons? O Do and Wh (elements 119 and 137 on special periodic table) O Wf and Bd (elements 148 and 146 on special periodic table) O Hr and Ba (elements 120 and 56 on special periodic table) O Wh and Rb (elements 137 and 37 on special periodic table)
ChapterU2: Smells: Molecular Structure And Properties
Section: Chapter Questions
Problem 6STP
Related questions
Question
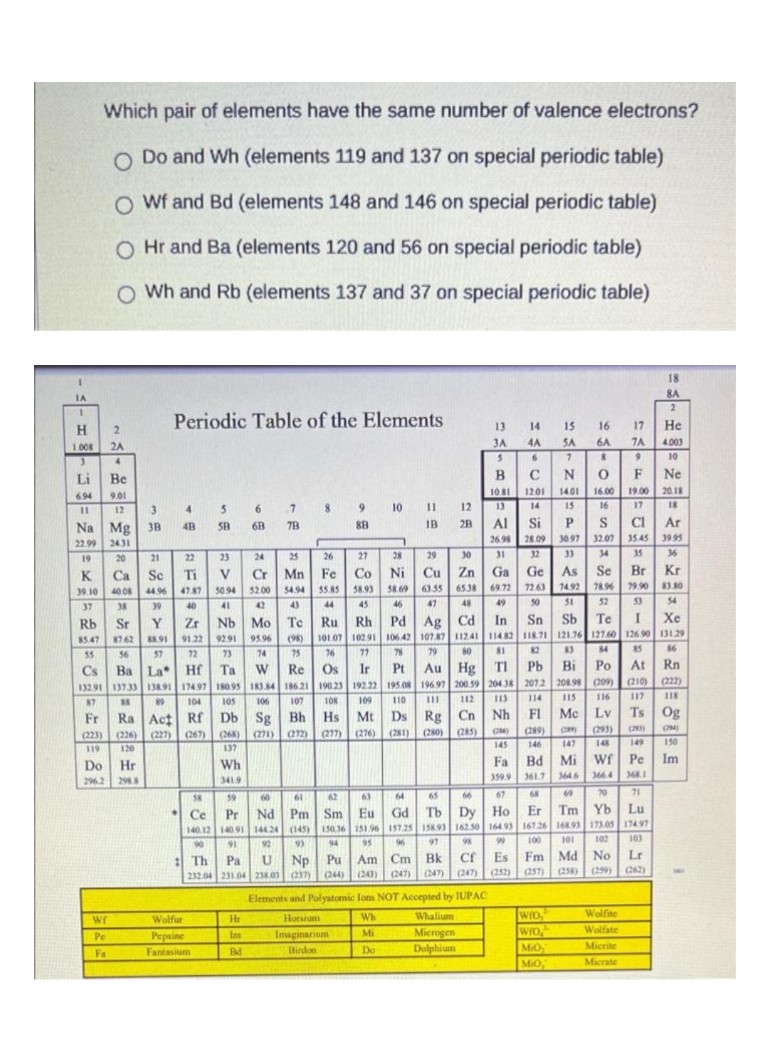
Transcribed Image Text:Which pair of elements have the same number of valence electrons?
O Do and Wh (elements 119 and 137 on special periodic table)
O Wf and Bd (elements 148 and 146 on special periodic table)
O Hr and Ba (elements 120 and 56 on special periodic table)
O Wh and Rb (elements 137 and 37 on special periodic table)
18
8A
IA
Periodic Table of the Elements
He
H.
13
14
15
16
17
2A
3A
4A
SA
6A
7A
4.003
1.008
3.
Li
10
4.
Be
F
Ne
16.00
19.00
20.18
10.81
13
1201
1401
694
9.01
12
8.
10
11
12
14
15
16
17
18
Na Mg
Al
Si
CI
Ar
3B
4B
5B
6B
78
8B
IB
28
26.98
28.09
30.97
32.07
35.45
39.95
22.99
2431
26
27
28
29
30 31
32
33
34
35
36
19
20
21
22
23
24
25
Zn Ga
6538 69.72 263
47
K
Ca
Sc
Ti
V
Cr Mn
Fe
Co
Ni
Cu
Ge
As
Se
Br
Kr
44 96
52.00
54.94
55.85
58.93
58.69
1492
78.96
79.90
83.80
39.10
40.08
47.87
5094
39
42
43
44
45
46
48
49
50
51
52
53
54
37
38
40
41
Rb
Y
Nb
Mo
Te
Ru
Rh
Ag | Cd| In
Sn
Sb Te
Xe
Sr
Zr
(98)
101.07 10291 2 107.87 11241 114.82 I8.71 121.76 127.60 126 90 3129
11241 IA H
81
85.47
8762
8.91
91.22
9291
95.96
10642
55
57
72
73
74
75
76
77
78
79
80
82
83
84
85
86
56
Ir
Au Hg TI
195.08 196 97 200 39 204 320
Ba La Hf
Ta
Os
0192.22
Pt
Bi
Po
At
Rn
Cs
Re
132.91 13733 13891 17497 180 95 183.84 186.21 190.23 192.22 195.08 196.97 200.59 20438 2072 20898 209) 210)
117
(222)
112 113
Cn Nh FI Me Lv
(285) CM (289)
104
107
108
109
110
III
114
116
118
87
105
106
Ra Act
(226) (227)
Db Sg
Bh Hs
Ds Rg
Ts Og
Fr
Rf
Mt
(276)
(281)
(280)
(293)
(23)
(268)
137
(223)
267)
(271)
212) 277)
150
145
146
147
148
149
119
120
Bd Mi
Fa
359.9 361.7
wf Pe
Im
Do
Hr
Wh
364.6
364 3.I
296.2 298
3419
59
60
61
62
63
64
65
60
67
68
69
70
71
• Ce
Pr
Nd Pm Sm
Eu Gd
Tb
Dy Ho
Er
Tm|
Yb| Lu
140.12 140.91 144.24 (145) 15036 151.96 157.25 15893 16250 164.93 167.26 16893 173.05 17497
103
93
95
96
97
98
99
100
101
102
90
91
94
Cf Es Fm Md No
Lr
Np Pu
(243)
Th
Pa
Am Cm
Bk
232.04 231.04 238.03
044)
(247)
(247)
(247) (252)
257)
(258)
(29)
Elements and Polyatomic lons NOT Accepted by IUPAC
Whalium
WIO
WrO
Wolfite
Horsium
Imaginarium
we
Wolfur
Hr
Wh
Microgen
Dolphium
Wolfate
Pe
Pepsine
Im
Mi
Fantasium
Bd
Birdon
Do
MiO
Micrite
Fa
MIO,
Micrate
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Living By Chemistry: First Edition Textbook
Chemistry
ISBN:
9781559539418
Author:
Angelica Stacy
Publisher:
MAC HIGHER

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Living By Chemistry: First Edition Textbook
Chemistry
ISBN:
9781559539418
Author:
Angelica Stacy
Publisher:
MAC HIGHER

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning