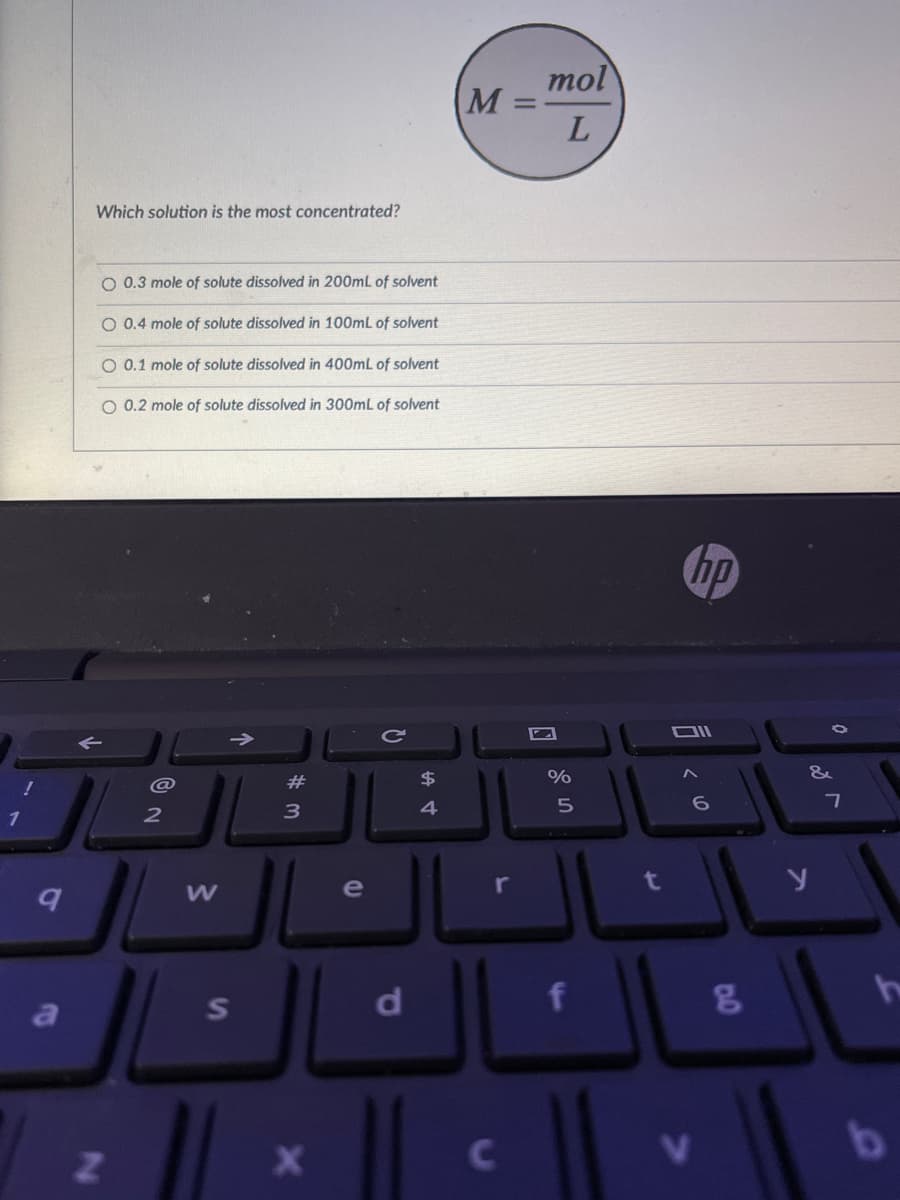
Which solution is the most concentrated? O 0.3 mole of solute dissolved in 200mL of solvent O 0.4 mole of solute dissolved in 100mL of solvent O 0.1 mole of solute dissolved in 400mL of solvent O 0.2 mole of solute dissolved in 300mL of solvent
Which solution is the most concentrated? O 0.3 mole of solute dissolved in 200mL of solvent O 0.4 mole of solute dissolved in 100mL of solvent O 0.1 mole of solute dissolved in 400mL of solvent O 0.2 mole of solute dissolved in 300mL of solvent
Chapter2: Crystallization
Section: Chapter Questions
Problem 3Q
Related questions
Question
Transcribed Image Text:mol
(M
Which solution is the most concentrated?
O 0.3 mole of solute dissolved in 200mL of solvent
O 0.4 mole of solute dissolved in 100mL of solvent
O 0.1 mole of solute dissolved in 400mL of solvent
O 0.2 mole of solute dissolved in 300mL of solvent
hp
23
$
%
&
@
3
5
2
e
d.
a
Cc
0O
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT



EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT



Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning