While working in a pharmaceutical laboratory, you need to prepare 0.250 L of a 1.60-M NaCl solution. What mass of NaCl would be required to prepare this solution? mass: How would you go about preparing the solution? Place the steps in order from first to last. First step Last step Answer Bank Dilute the solution, slowly adding water until the desired volume is reached. Partially fill the flask with water. Mix until NaCl dissolves completely. Measure out the desired amount of NaCl. Add the measured NaCl to the 0.250-L volumetric flask. g 6.0
While working in a pharmaceutical laboratory, you need to prepare 0.250 L of a 1.60-M NaCl solution. What mass of NaCl would be required to prepare this solution? mass: How would you go about preparing the solution? Place the steps in order from first to last. First step Last step Answer Bank Dilute the solution, slowly adding water until the desired volume is reached. Partially fill the flask with water. Mix until NaCl dissolves completely. Measure out the desired amount of NaCl. Add the measured NaCl to the 0.250-L volumetric flask. g 6.0
Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter4: Types Of Chemical Reactions And Solution Stoichiometry
Section: Chapter Questions
Problem 20Q: A student wants to prepare 1.00 L of a 1.00-M solution of NaOH (molar mass = 40.00 g/mol). If solid...
Related questions
Question
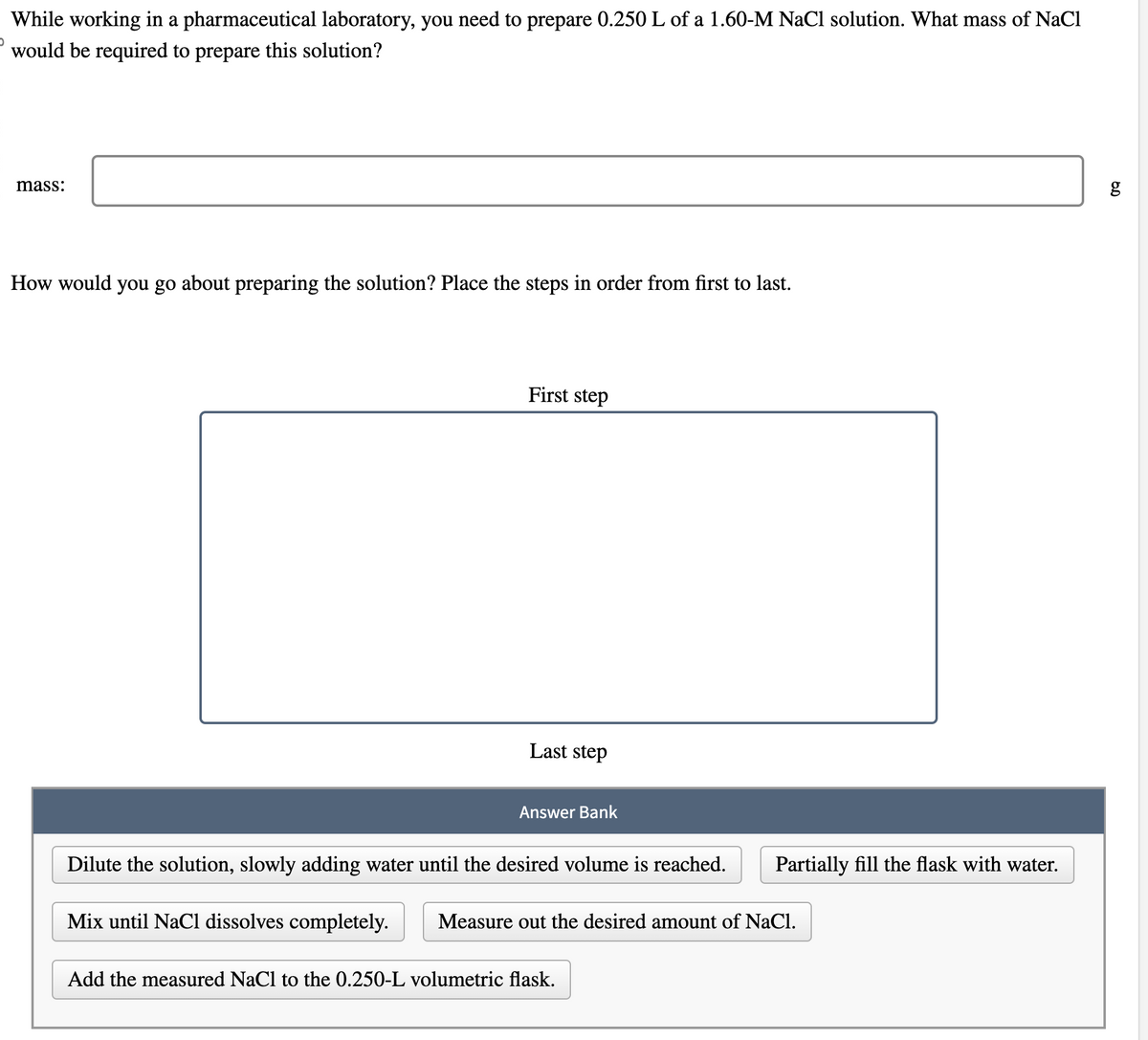
Transcribed Image Text:While working in a pharmaceutical laboratory, you need to prepare 0.250 L of a 1.60-M NaCl solution. What mass of NaCl
would be required to prepare this solution?
mass:
How would you go about preparing the solution? Place the steps in order from first to last.
First step
Last step
Answer Bank
Dilute the solution, slowly adding water until the desired volume is reached.
Partially fill the flask with water.
Mix until NaCl dissolves completely.
Measure out the desired amount of NaCl.
Add the measured NaCl to the 0.250-L volumetric flask.
g
6.0
AI-Generated Solution
Unlock instant AI solutions
Tap the button
to generate a solution
Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning