Why are atoms electrically neutral? O they have more electrons than protons O they have less electrons than protons they have the same number of electrons as neutrons they have the same number of electrons as protons O they have the same number of neutrons as protons
Why are atoms electrically neutral? O they have more electrons than protons O they have less electrons than protons they have the same number of electrons as neutrons they have the same number of electrons as protons O they have the same number of neutrons as protons
Chemistry for Today: General, Organic, and Biochemistry
9th Edition
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Chapter2: Atoms And Molecules
Section: Chapter Questions
Problem 2.81E: Atoms are electrically neutral. This means that an atom will contain a.more protons than neutrons....
Related questions
Question
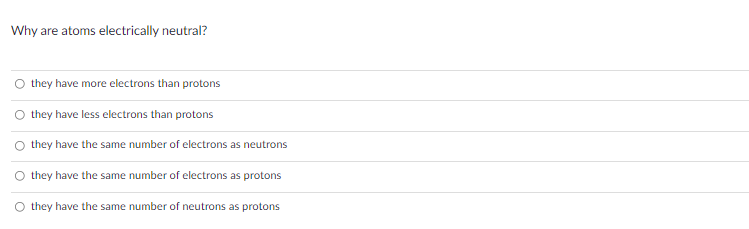
Transcribed Image Text:Why are atoms electrically neutral?
O they have more electrons than protons
O they have less electrons than protons
they have the same number of electrons as neutrons
they have the same number of electrons as protons
O they have the same number of neutrons as protons
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning


Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning


Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning