Why is light of at least a certain frequency needed to cause the photoelectric effect? O A certain quantity of energy is necessary to eject electrons from a metal A certain number of photons is necessary to eject electrons from a metal O A certain temperature is necessary to eject electrons from a metal O A certain number of atoms is necessary to eject electrons from a metal O A certain speed of light is necessary to eject electrons from a metal
Why is light of at least a certain frequency needed to cause the photoelectric effect? O A certain quantity of energy is necessary to eject electrons from a metal A certain number of photons is necessary to eject electrons from a metal O A certain temperature is necessary to eject electrons from a metal O A certain number of atoms is necessary to eject electrons from a metal O A certain speed of light is necessary to eject electrons from a metal
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter4: Introduction To Quantum Mechanics
Section: Chapter Questions
Problem 50AP
Related questions
Question
Transcribed Image Text:quizzes/982684
OneNote
101 Chem101
Tophat
* USU Tutoring
Scholarships :)
Morgage Amer
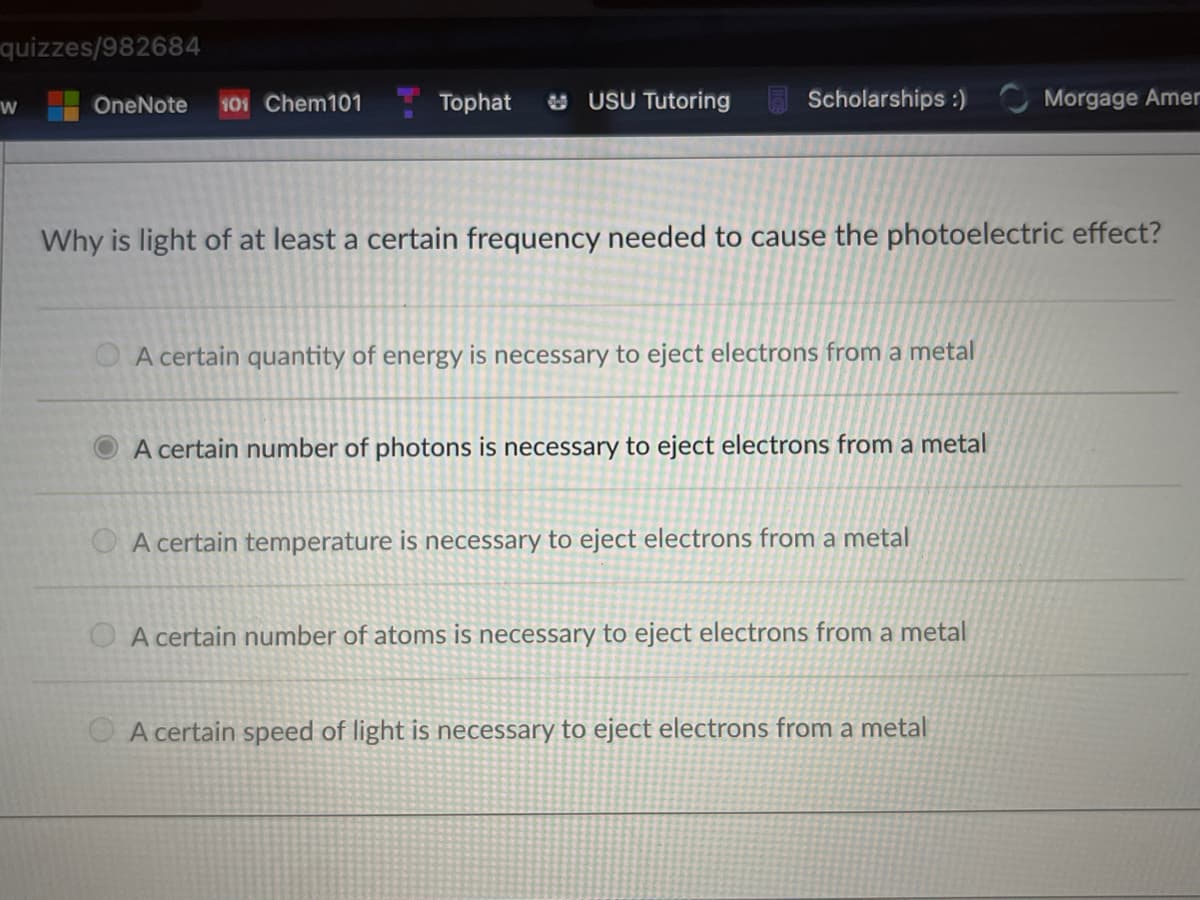
Why is light of at least a certain frequency needed to cause the photoelectric effect?
O A certain quantity of energy is necessary to eject electrons from a metal
A certain number of photons is necessary to eject electrons from a metal
A certain temperature is necessary to eject electrons from a metal
O A certain number of atoms is necessary to eject electrons from a metal
O A certain speed of light is necessary to eject electrons from a metal
Expert Solution
Step 1
Photoelectric effect :- J.J Thomson observed that when a photon of having certain frequency strikes the surface of metal then the electrons are ejected from the metal surface. This phenomenon of ejection of electron via photon is known as photoelectric effect and the ejected electron are called as photoelectrons.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning