Why is the compound shown not considered aromatic ev though it has 3 double bonds in conjugation and a 6- membered ring? I.Ilt is not planar II.It does not contain 4n+2 pi electrons III.It does not have a continuous cyclic system of pi orbita A) Ionly B) I and III III only
Why is the compound shown not considered aromatic ev though it has 3 double bonds in conjugation and a 6- membered ring? I.Ilt is not planar II.It does not contain 4n+2 pi electrons III.It does not have a continuous cyclic system of pi orbita A) Ionly B) I and III III only
Chapter16: Chemistry Of Benzene: Electrophilic Aromatic Substitution
Section16.SE: Something Extra
Problem 25VC: The following molecular model of a dimethyl-substituted biphenyl represents the lowest-energy...
Related questions
Question
100%
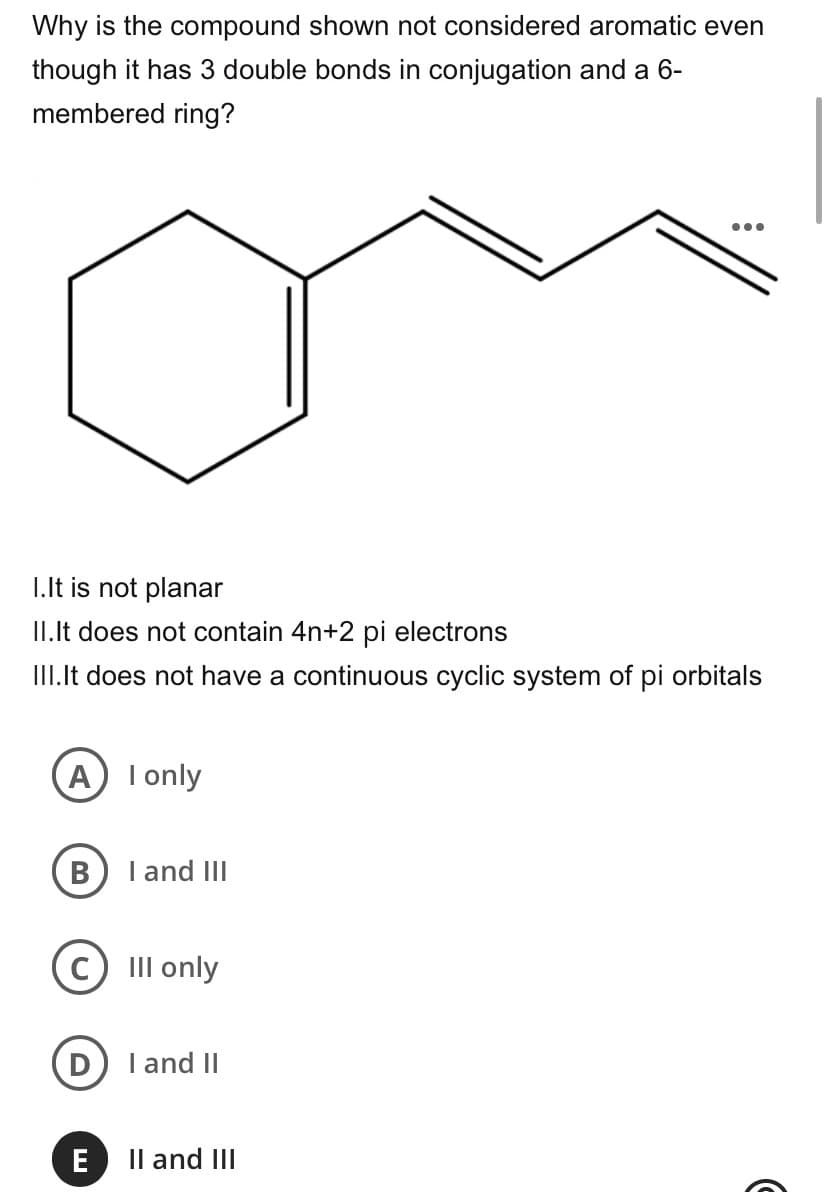
Transcribed Image Text:Why is the compound shown not considered aromatic even
though it has 3 double bonds in conjugation and a 6-
membered ring?
•..
I.It is not planar
II.Ilt does not contain 4n+2 pi electrons
III.It does not have a continuous cyclic system of pi orbitals
A I only
B
I and III
III only
I and II
E
Il and III
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Recommended textbooks for you


Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning


Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning