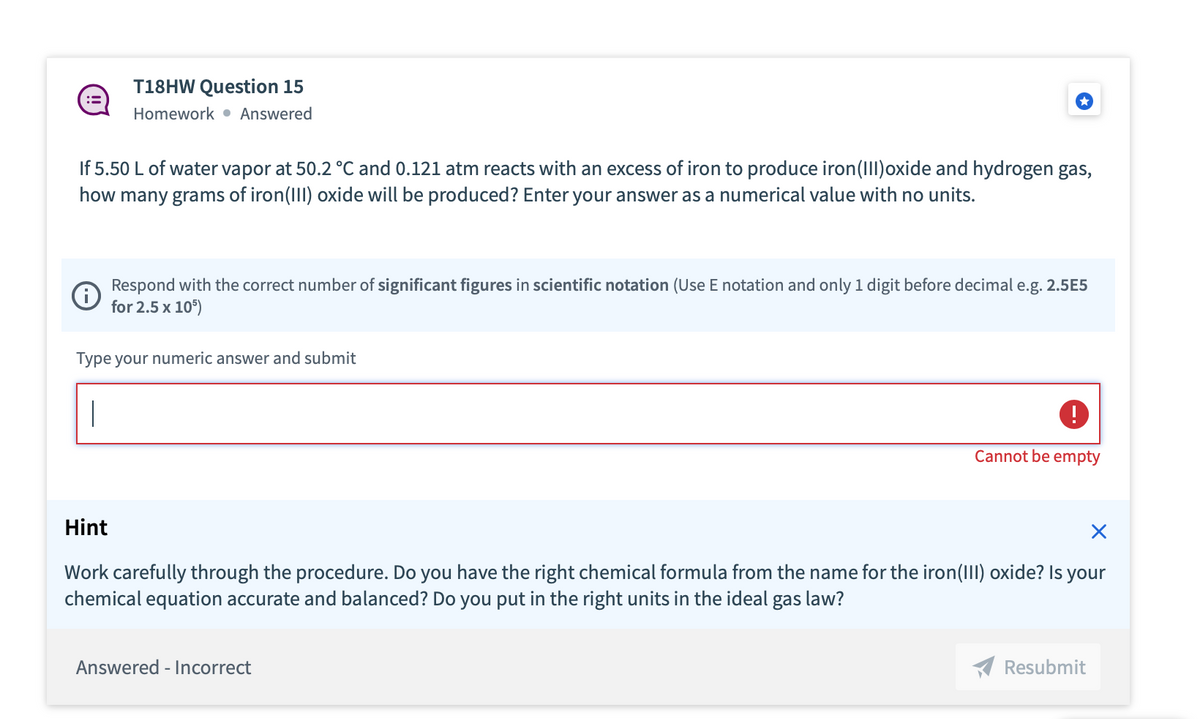
work If 5.50 L of water vapor at 50.2 °C and 0.121 atm reacts with an excess of iron to produce iron (III)oxide and hydrogen gas, how many grams of iron (III) oxide will be produced? Enter your answer as a numerical value with no units. Respond with the correct number of significant figures in scientific notation (Use E notation and only 1 digit before decimal e.g. 2.5E5 for 2.5 x 10") Type your numeric answer and submit Cannot be empty
work If 5.50 L of water vapor at 50.2 °C and 0.121 atm reacts with an excess of iron to produce iron (III)oxide and hydrogen gas, how many grams of iron (III) oxide will be produced? Enter your answer as a numerical value with no units. Respond with the correct number of significant figures in scientific notation (Use E notation and only 1 digit before decimal e.g. 2.5E5 for 2.5 x 10") Type your numeric answer and submit Cannot be empty
Chapter5: Gases
Section: Chapter Questions
Problem 74E: Urea (H2NCONH2) is used extensively as a nitrogen source in fertilizers. It is produced commercially...
Related questions
Question
Transcribed Image Text::=
T18HW Question 15
Homework Answered
If 5.50 L of water vapor at 50.2 °C and 0.121 atm reacts with an excess of iron to produce iron (III)oxide and hydrogen gas,
how many grams of iron(III) oxide will be produced? Enter your answer as a numerical value with no units.
℗
Respond with the correct number of significant figures in scientific notation (Use E notation and only 1 digit before decimal e.g. 2.5E5
for 2.5 x 105)
Type your numeric answer and submit
|
!
Cannot be empty
Hint
X
Work carefully through the procedure. Do you have the right chemical formula from the name for the iron(III) oxide? Is your
chemical equation accurate and balanced? Do you put in the right units in the ideal gas law?
Answered - Incorrect
Resubmit
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning