
Chemistry for Engineering Students
4th Edition
ISBN: 9781337398909
Author: Lawrence S. Brown, Tom Holme
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 5, Problem 5.84PAE
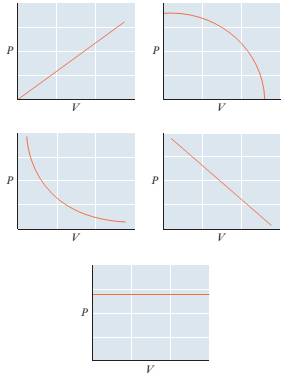
84 Consider a sample of an ideal gas with n and T held constant. Which of the graphs below represents the proper relationship between P and V? How would the graph differ for a sample with a larger number of moles?
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Chapter 5 Solutions
Chemistry for Engineering Students
Ch. 5 - Prob. 1COCh. 5 - Prob. 2COCh. 5 - Prob. 3COCh. 5 - Prob. 4COCh. 5 - perform stoichiometric ca1cu1uions for reactions...Ch. 5 - Prob. 6COCh. 5 - Prob. 7COCh. 5 - Prob. 8COCh. 5 - Prob. 9COCh. 5 - Prob. 10CO
Ch. 5 - Prob. 11COCh. 5 - Prob. 5.1PAECh. 5 - Prob. 5.2PAECh. 5 - What possible uses exist for the natural gas...Ch. 5 - How does hydraulic fracturing differ from...Ch. 5 - Prob. 5.5PAECh. 5 - Use the internet to determine what areas of the...Ch. 5 - Prob. 5.7PAECh. 5 - Prob. 5.8PAECh. 5 - Prob. 5.9PAECh. 5 - Prob. 5.10PAECh. 5 - Prob. 5.11PAECh. 5 - 5.12 Water has a density that is 13.6 times less...Ch. 5 - 5.13 Water has a density that is 13.6 times less...Ch. 5 - Prob. 5.14PAECh. 5 - 5.15 Gas pressure can be expressed in units of mm...Ch. 5 - 5.16 If the atmospheric pressure is 97.4 kPa, how...Ch. 5 - Prob. 5.17PAECh. 5 - 5.18 When helium escapes from a balloon, the...Ch. 5 - 5.19 A sample of CO2 gas has a pressure of 56.5 mm...Ch. 5 - Prob. 5.20PAECh. 5 - Prob. 5.21PAECh. 5 - Prob. 5.22PAECh. 5 - 5.23 A gas bubble forms inside a vat containing a...Ch. 5 - 5.24 A bicycle tire is inflated to a pressure of...Ch. 5 - 5.25 A balloon filled with helium has a volume of...Ch. 5 - 5.26 How many moles of an ideal gas are there if...Ch. 5 - 5.27 A newly discovered gas has a density of 2.39...Ch. 5 - 5.28 Calculate the mass of each of the following...Ch. 5 - 5.29 What are the densities of the following gases...Ch. 5 - Prob. 5.30PAECh. 5 - 5.31 A cylinder is filled with toxic COS gas to a...Ch. 5 - 5.32 Cylinders of compressed gases are often...Ch. 5 - Prob. 5.33PAECh. 5 - 5.34 Define the term mole fractionCh. 5 - Prob. 5.35PAECh. 5 - 36 What is the total pressure exerted by a mixture...Ch. 5 - Prob. 5.37PAECh. 5 - 38 For a gas sample whose total pressure is 740...Ch. 5 - 39 A sample containing only NO2 and SO2, has a...Ch. 5 - Prob. 5.40PAECh. 5 - 41 A sample of a smokestack emission was collected...Ch. 5 - 42 Air is often dry air, ignoring the water mole...Ch. 5 - 43 In an experiment, a mixture of gases occupies a...Ch. 5 - Prob. 5.44PAECh. 5 - Prob. 5.45PAECh. 5 - Prob. 5.46PAECh. 5 - 47 HCl(g) reacts with ammonia gas, NH3(g), to form...Ch. 5 - 48 Hydrogen gas is generated when acids come into...Ch. 5 - Prob. 5.49PAECh. 5 - 50 The first step in processing zinc metal from...Ch. 5 - 51 What volume of oxygen at 24 C and 0.88 atm is...Ch. 5 - 52 If tetraborane, B4H10, is treated with pure...Ch. 5 - 53 N2O5is an unstable gas that decomposes...Ch. 5 - 54 One way to generate oxygen is to heat potassium...Ch. 5 - 55 Ammonia is not the only possible fertilizer....Ch. 5 - 56 Consider the following reaction:...Ch. 5 - 57 What volume of hydrogen gas, in liters, is...Ch. 5 - 58 Magnesium will burn in air to form both Mg3N2...Ch. 5 - 59 During a collision, automobile air bags are...Ch. 5 - 60 Automakers are always investigating reactions...Ch. 5 - 61 As one step in its purification, nickel metal...Ch. 5 - 62 Ammonium dinitramide (ADN), NH4N(NO2)2, was...Ch. 5 - Prob. 5.63PAECh. 5 - Prob. 5.64PAECh. 5 - Prob. 5.65PAECh. 5 - Prob. 5.66PAECh. 5 - Prob. 5.67PAECh. 5 - Prob. 5.68PAECh. 5 - Prob. 5.69PAECh. 5 - Prob. 5.70PAECh. 5 - Prob. 5.71PAECh. 5 - Prob. 5.72PAECh. 5 - Prob. 5.73PAECh. 5 - Prob. 5.74PAECh. 5 - Prob. 5.75PAECh. 5 - Prob. 5.76PAECh. 5 - Prob. 5.77PAECh. 5 - Prob. 5.78PAECh. 5 - Prob. 5.79PAECh. 5 - Prob. 5.80PAECh. 5 - Prob. 5.81PAECh. 5 - 82 Why do heavier gases move more slowly than...Ch. 5 - 83 Suppose that speed distribution for each of the...Ch. 5 - 84 Consider a sample of an ideal gas with n and T...Ch. 5 - Prob. 5.85PAECh. 5 - Prob. 5.86PAECh. 5 - Prob. 5.87PAECh. 5 - 88 Liquid oxygen for use as a rocket fuel can be...Ch. 5 - 89 A number of compounds containing the heavier...Ch. 5 - Prob. 5.90PAECh. 5 - 91 A 0.2500-g sample of an Al-Zn alloy reacts with...Ch. 5 - Prob. 5.92PAECh. 5 - 93 The complete combustion of octane can be used...Ch. 5 - 94 Mining engineers often have to deal with gases...Ch. 5 - 95 Some engineering designs call for the use of...Ch. 5 - Prob. 5.96PAECh. 5 - 97 Homes in rural areas where natural gas service...Ch. 5 - Prob. 5.98PAECh. 5 - 99 Pure gaseous nitrogen dioxide (NO2) cannot be...Ch. 5 - Prob. 5.100PAECh. 5 - Prob. 5.101PAECh. 5 - 102 A mixture of helium and neon gases has a...Ch. 5 - Prob. 5.103PAECh. 5 - 104 When a 0.817-g sample of a copper oxide is...Ch. 5 - 105 The decomposition of mercury(II) thiocyanate...Ch. 5 - Prob. 5.106PAECh. 5 - 107 A soft drink can’s label indicates that the...Ch. 5 - Prob. 5.108PAECh. 5 - 109 An ore sample with a mass of 670 kg contains...Ch. 5 - Prob. 5.110PAECh. 5 - 111 Consider a room that is 14ft20ft wih an 8-ft...Ch. 5 - Prob. 5.112PAECh. 5 - 113 A 0.0125-g sample of a gas with an empirical...Ch. 5 - Prob. 5.114PAE
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Explain why the plot of PV for CO2 differs from that of an ideal gas.arrow_forwardUnder which of the following sets of conditions does a real gas behave most like an ideal gas, and for which conditions is a real gas expected to deviate from ideal behavior? Explain. (a) high pressure, small volume (b) high temperature, low pressure (c) low temperature, high pressurearrow_forwardFor a given amount of gas showing ideal behavior, draw labeled graphs of: (a) the variation of P with V (b) the variation of V with T (C) the variation of P with T (d) the variation of 1P with Varrow_forward
- Consider a 5.00-L tank containing 375 g of Ar at a temperature of 25 C. (a) Calculate the pressure in the tank using both the ideal gas law and the van der Waals equation. (b) Which correction term, a(n/V)2 or bn, has the greatest influence on the pressure of this system?arrow_forwardDescribe the factors responsible for the deviation of the behavior of real gases from that of an ideal gas.arrow_forwardDescribe what happens o the average kinetic energy of ideal gas molecules when the conditions are changed as follows: (a) The pressure of the gas is increased by reducing the volume at constant temperature. (b) The pressure of the gas is increased by increasing the temperature at constant volume. (c) The average velocity of the molecules is increased by a factor of 2.arrow_forward
- What is the temperature of an 11.2-L sample of carbon monoxide, CO, at 744 torr if it occupies 13.3 L at 55 C and 74-4 torr?arrow_forwardIf you have a 150-L cylinder filled with chlorine gas to a density of 2.8 g/L, how many moles of chlorine would you need to add to the cylinder? If you were to double the temperature of the cylinder, would the gas density change?arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning Chemistry for Engineering StudentsChemistryISBN:9781285199023Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781285199023Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax- Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
 Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:9781285199023
Author:Lawrence S. Brown, Tom Holme
Publisher:Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:OpenStax

Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning
Step by Step Stoichiometry Practice Problems | How to Pass ChemistryMole Conversions Made Easy: How to Convert Between Grams and Moles; Author: Ketzbook;https://www.youtube.com/watch?v=b2raanVWU6c;License: Standard YouTube License, CC-BY