Write a balanced equation that describes each of the following chemical reaction. 1. Acetylene gas, C2H2, burns in air forming gaseous carbon dioxide and, CO2, and water. Answer: 2C2H2 + 502 → 4CO2 + 2H20 (double displacement/Combustion reaction) 2. Acid rain, a solution of sulfurous acid, H&PO4(aq), forms when sulfur dioxide reacts, SO2, reacts with water. 3. Water vapor reacts with sodium metal to produce gaseous hydrogen, H2, and solid sodium hydroxide, NaOH. 4. Gaseous water and hot carbon react to form gaseous hydrogen, H2, gaseous carbon dioxide. 5. Ca3(PO4)2 + H3PO4 → Ca(H2PO.)2 6. Ag + H2S + O2 Ag2S + H2O nglish (United States) O Accessibility Good to go
Write a balanced equation that describes each of the following chemical reaction. 1. Acetylene gas, C2H2, burns in air forming gaseous carbon dioxide and, CO2, and water. Answer: 2C2H2 + 502 → 4CO2 + 2H20 (double displacement/Combustion reaction) 2. Acid rain, a solution of sulfurous acid, H&PO4(aq), forms when sulfur dioxide reacts, SO2, reacts with water. 3. Water vapor reacts with sodium metal to produce gaseous hydrogen, H2, and solid sodium hydroxide, NaOH. 4. Gaseous water and hot carbon react to form gaseous hydrogen, H2, gaseous carbon dioxide. 5. Ca3(PO4)2 + H3PO4 → Ca(H2PO.)2 6. Ag + H2S + O2 Ag2S + H2O nglish (United States) O Accessibility Good to go
Chemistry: An Atoms First Approach
2nd Edition
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Steven S. Zumdahl, Susan A. Zumdahl
Chapter8: Gases
Section: Chapter Questions
Problem 155CP: Methane (CH4) gas flows into a combustion chamber at a rate of 200. L/min at 1.50 atm and ambient...
Related questions
Question
Transcribed Image Text:AaBbC AABBCCL
ID AaBbCcD A
BIU abe x, x
A - A
1 Normal
1 No Spac. Heading 1
Heading 2
Title
Subtitle
Font
Paragraph
Styles
M 1
4 I5
12 13 . 14 15 16 .
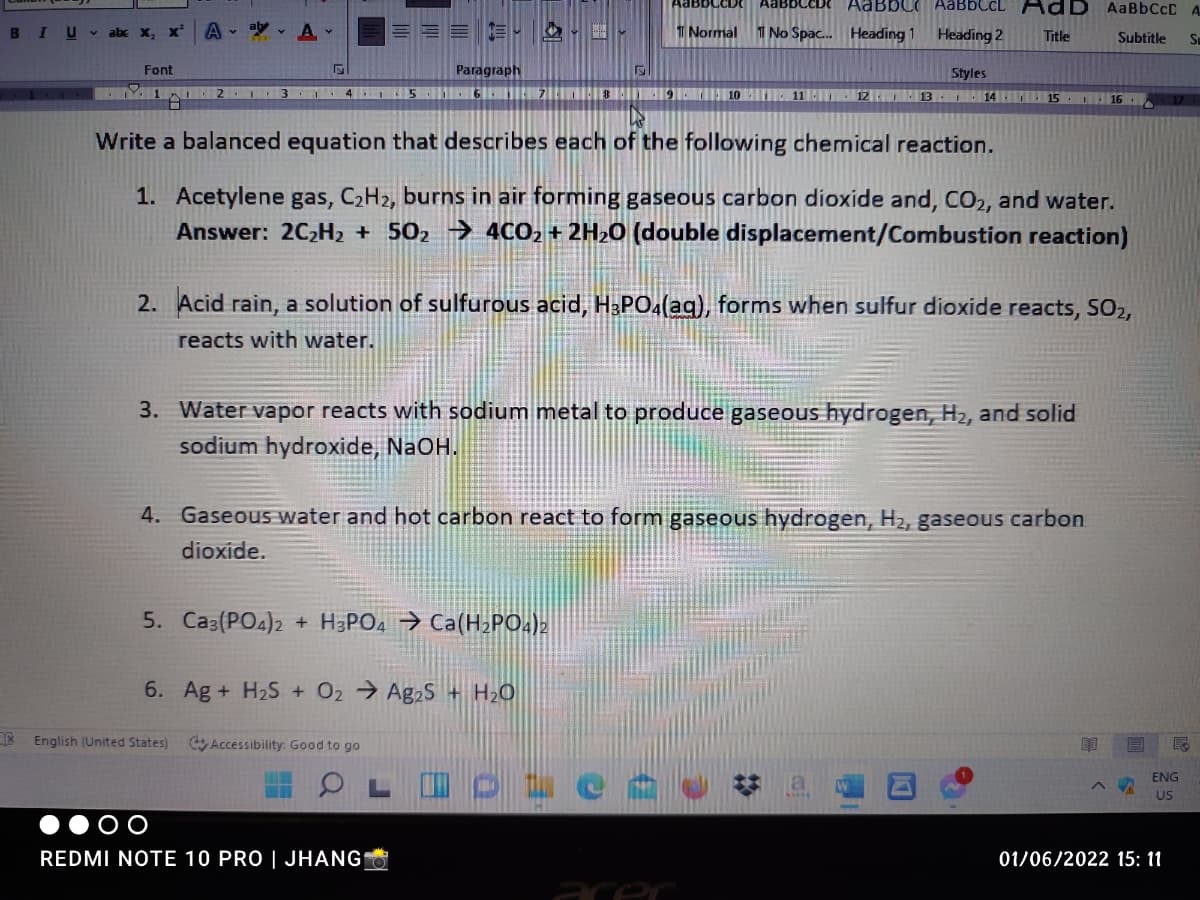
Write a balanced equation that describes each of the following chemical reaction.
1. Acetylene gas, C2H2, burns in air forming gaseous carbon dioxide and, CO2, and water.
Answer: 2C2H2 + 502 → 4CO2 + 2H20 (double displacement/Combustion reaction)
2. Acid rain, a solution of sulfurous acid, H&PO4(aq), forms when sulfur dioxide reacts, SO2,
reacts with water.
3. Water vapor reacts with sodium metal to produce gaseous hydrogen, H2, and solid
sodium hydroxide, NaOH.
4. Gaseous water and hot carbon react to form gaseous hydrogen, H2, gaseous carbon
dioxide.
5. Ca3(PO4)2 + H3PO4 → Ca(H2PO.)2
6. Ag + H2S + O2 Ag2S + H2O
English (United States)
Accessibility: Good to go
ENG
US
REDMI NOTE 10 PRO | JHANG
01/06/2022 15: 11
%23
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning
