Chemistry by OpenStax (2015-05-04)
1st Edition
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Chapter5: Thermochemistry
Section: Chapter Questions
Problem 52E: Before the introduction of chlorofluorocarbons, sulfur dioxide (enthalpy of vaporization, 6.00...
Related questions
Question
Transcribed Image Text:Question 19 of 50
< >
-/1 E
View Policies
Current Attempt in Progress
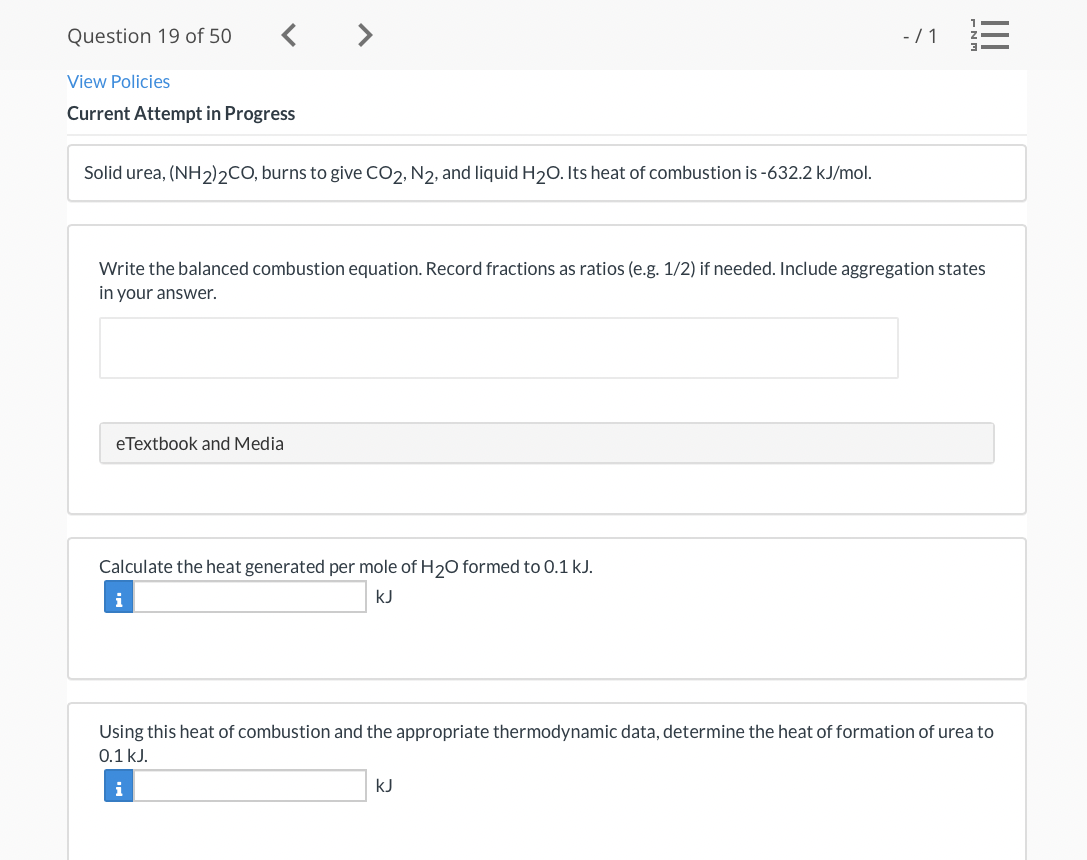
Solid urea, (NH2)2CO, burns to give CO2, N2, and liquid H20. Its heat of combustion is -632.2 kJ/mol.
Write the balanced combustion equation. Record fractions as ratios (e.g. 1/2) if needed. Include aggregation states
in your answer.
eTextbook and Media
Calculate the heat generated per mole of H20 formed to 0.1 kJ.
i
kJ
Using this heat of combustion and the appropriate thermodynamic data, determine the heat of formation of urea to
0.1 kJ.
i
kJ
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781285199030
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781285199030
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning