Write the chemical reaction that occurs between the two reacting species. Include physical states. molecular equation: What volume of the solution is needed to completely react all 8.05 g of zinc powder? volume: L Calculate the molarity of the Zn²+ ions in solution after the reaction. concentration: M - TOOLS
Write the chemical reaction that occurs between the two reacting species. Include physical states. molecular equation: What volume of the solution is needed to completely react all 8.05 g of zinc powder? volume: L Calculate the molarity of the Zn²+ ions in solution after the reaction. concentration: M - TOOLS
General Chemistry - Standalone book (MindTap Course List)
11th Edition
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Chapter4: Chemical Reactions
Section: Chapter Questions
Problem 4.97QP: Nickel(II) sulfate solution reacts with sodium hydroxide solution to produce a precipitate of...
Related questions
Question
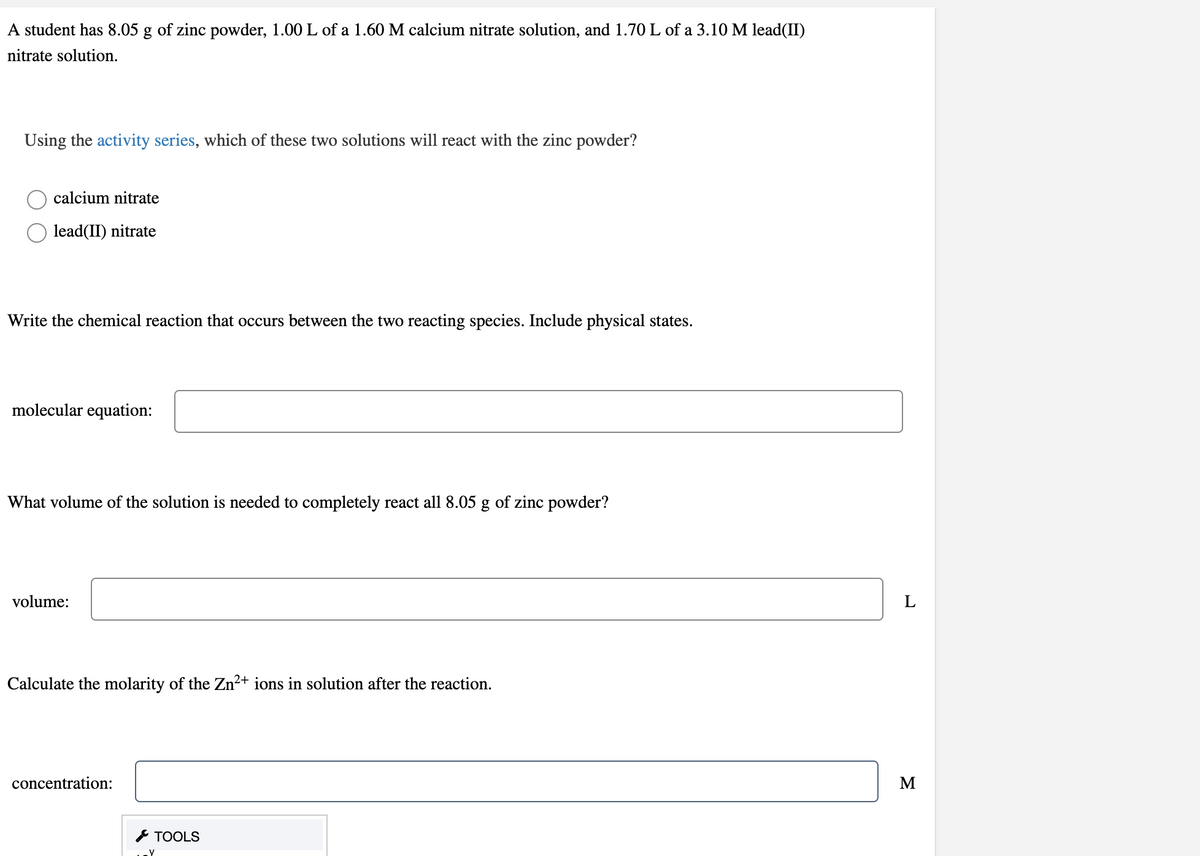
Transcribed Image Text:A student has 8.05 g of zinc powder, 1.00 L of a 1.60 M calcium nitrate solution, and 1.70 L of a 3.10 M lead(II)
nitrate solution.
Using the activity series, which of these two solutions will react with the zinc powder?
calcium nitrate
lead(II) nitrate
Write the chemical reaction that occurs between the two reacting species. Include physical states.
molecular equation:
What volume of the solution is needed to completely react all 8.05 g of zinc powder?
volume:
Calculate the molarity of the Zn²+ ions in solution after the reaction.
concentration:
M
* TOOLS
y
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 3 images

Recommended textbooks for you

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning