Write the mass-balance expressions for a solution that is a. 0.15 M in HF. 0.15 M HF F b. 0.16 M in CH3 NH2. CH,NH, + CH,NH, 0.16 M %3! c. saturated with Ag,S. %3D
Write the mass-balance expressions for a solution that is a. 0.15 M in HF. 0.15 M HF F b. 0.16 M in CH3 NH2. CH,NH, + CH,NH, 0.16 M %3! c. saturated with Ag,S. %3D
Chemistry: Matter and Change
1st Edition
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Chapter17: Chemical Equilibrium
Section17.3: Using Equilibrium Constants
Problem 20PP
Related questions
Question
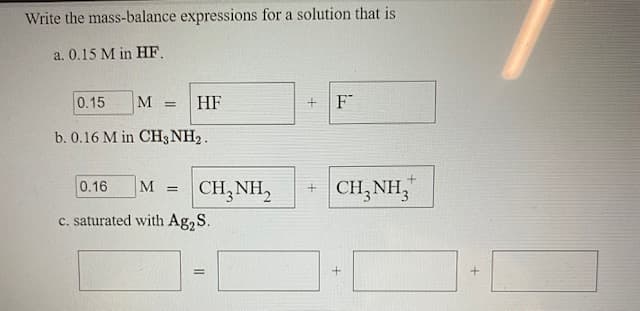
Transcribed Image Text:Write the mass-balance expressions for a solution that is
a. 0.15 M in HF.
0.15
M
HF
F
b. 0.16 M in CH3 NH2.
+ CH,NH,
"
0.16
M
CH,NH,
c. saturated with Ag,S.
%3D
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Recommended textbooks for you

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co


Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co


Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax