Write the orbital diagram for the ions of Cr and Fe* and O applying Pauli Exclusion principle and Hund's rules. (you can use noble-gas core configuration to write out the (al orbital diagrams) (b) Identify their magnetic properties (paramagnetic or diamagnetic). (c) Which one has the highest ionic radii, and which one has the lowest? Justify your answer
Write the orbital diagram for the ions of Cr and Fe* and O applying Pauli Exclusion principle and Hund's rules. (you can use noble-gas core configuration to write out the (al orbital diagrams) (b) Identify their magnetic properties (paramagnetic or diamagnetic). (c) Which one has the highest ionic radii, and which one has the lowest? Justify your answer
Chemistry: Principles and Practice
3rd Edition
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Chapter8: The Periodic Table: Structure And Trends
Section: Chapter Questions
Problem 8.11QE
Related questions
Question
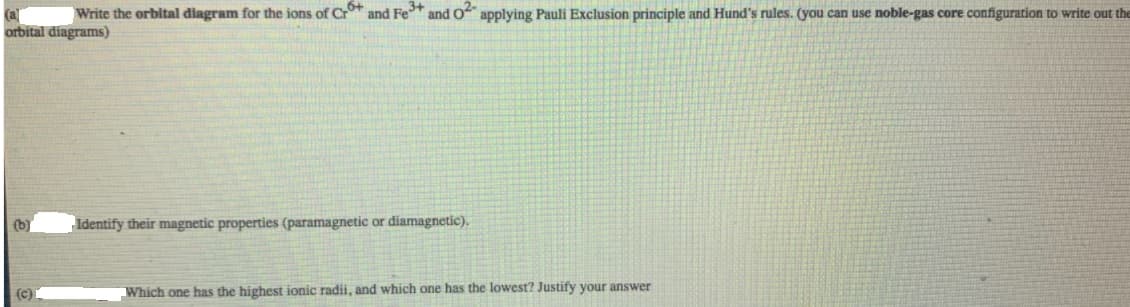
Transcribed Image Text:Write the orbital diagram for the ions of Cr and Fe* and O applying Pauli Exclusion principle and Hund's rules. (you can use noble-gas core configuration to write out the
(al
orbital diagrams)
(b)
Identify their magnetic properties (paramagnetic or diamagnetic).
(c)
Which one has the highest ionic radii, and which one has the lowest? Justify your answer
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 3 images

Recommended textbooks for you

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning


Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning