Write the product of the two reactions shown below. In the first step indicate if the product is meso or is formed as a racemic mixture. Indicate whether final structure in the second reaction is cis or trans. Br-Br H LDA, THF solvent
Write the product of the two reactions shown below. In the first step indicate if the product is meso or is formed as a racemic mixture. Indicate whether final structure in the second reaction is cis or trans. Br-Br H LDA, THF solvent
Chapter6: Valence Electrons (redux)
Section: Chapter Questions
Problem 5EQ
Related questions
Question
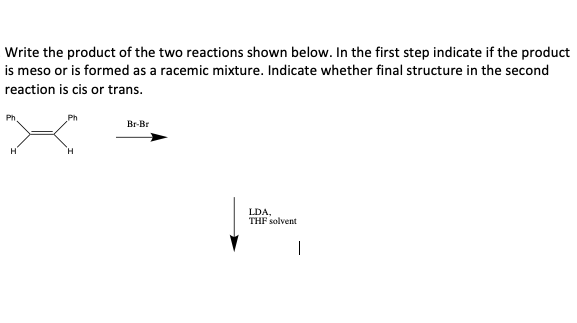
Transcribed Image Text:Write the product of the two reactions shown below. In the first step indicate if the product
is meso or is formed as a racemic mixture. Indicate whether final structure in the second
reaction is cis or trans.
Ph
Br-Br
H.
LDA,
THF solvent
|
Expert Solution
Step 1
Br2 adds to an alkene in an anti manner. The reaction proceeds by the formation of bromonium ion. In case of cis alkene, both cis and trans products are formed.
LDA is used to abstract the H+ resulting in the formation of alkene.
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT


Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT