Writing names and formulas for ionic compounds containing non- common monatomic ions 1. What this video FIRST : https://youtu.be/Rq0A-AHDB74 (10 min: watch the ENTIRE video) 2. Complete the following table: Write the names of the ions on the line below the ion formula (first row and column) b. (*) Find the correct ion formula based on the name of the ion a. Combine the cations with the anions to form a charge balanced (charge neutral) ionic compound d. Write the correct ionic compound formula and name in the table (see example in first cell of table) C. Br: N3-: H: _bromide_ Hydride Nitride Example: FeCla Iron(II) bromide Fe3+ : _Iron(III) CuaN CuH Copper(i) Nitride Copper Hydride Cut : CUB. Cuprous Copper Pb2+ : Lead Zn2+ : Zinc l1) :(„) Silver ion Mn3+ : Manganesc (11) Fe Brz Fe2+ : Iron Sn4+ :
Writing names and formulas for ionic compounds containing non- common monatomic ions 1. What this video FIRST : https://youtu.be/Rq0A-AHDB74 (10 min: watch the ENTIRE video) 2. Complete the following table: Write the names of the ions on the line below the ion formula (first row and column) b. (*) Find the correct ion formula based on the name of the ion a. Combine the cations with the anions to form a charge balanced (charge neutral) ionic compound d. Write the correct ionic compound formula and name in the table (see example in first cell of table) C. Br: N3-: H: _bromide_ Hydride Nitride Example: FeCla Iron(II) bromide Fe3+ : _Iron(III) CuaN CuH Copper(i) Nitride Copper Hydride Cut : CUB. Cuprous Copper Pb2+ : Lead Zn2+ : Zinc l1) :(„) Silver ion Mn3+ : Manganesc (11) Fe Brz Fe2+ : Iron Sn4+ :
World of Chemistry, 3rd edition
3rd Edition
ISBN:9781133109655
Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Chapter4: Nomenclature
Section: Chapter Questions
Problem 52A
Related questions
Question
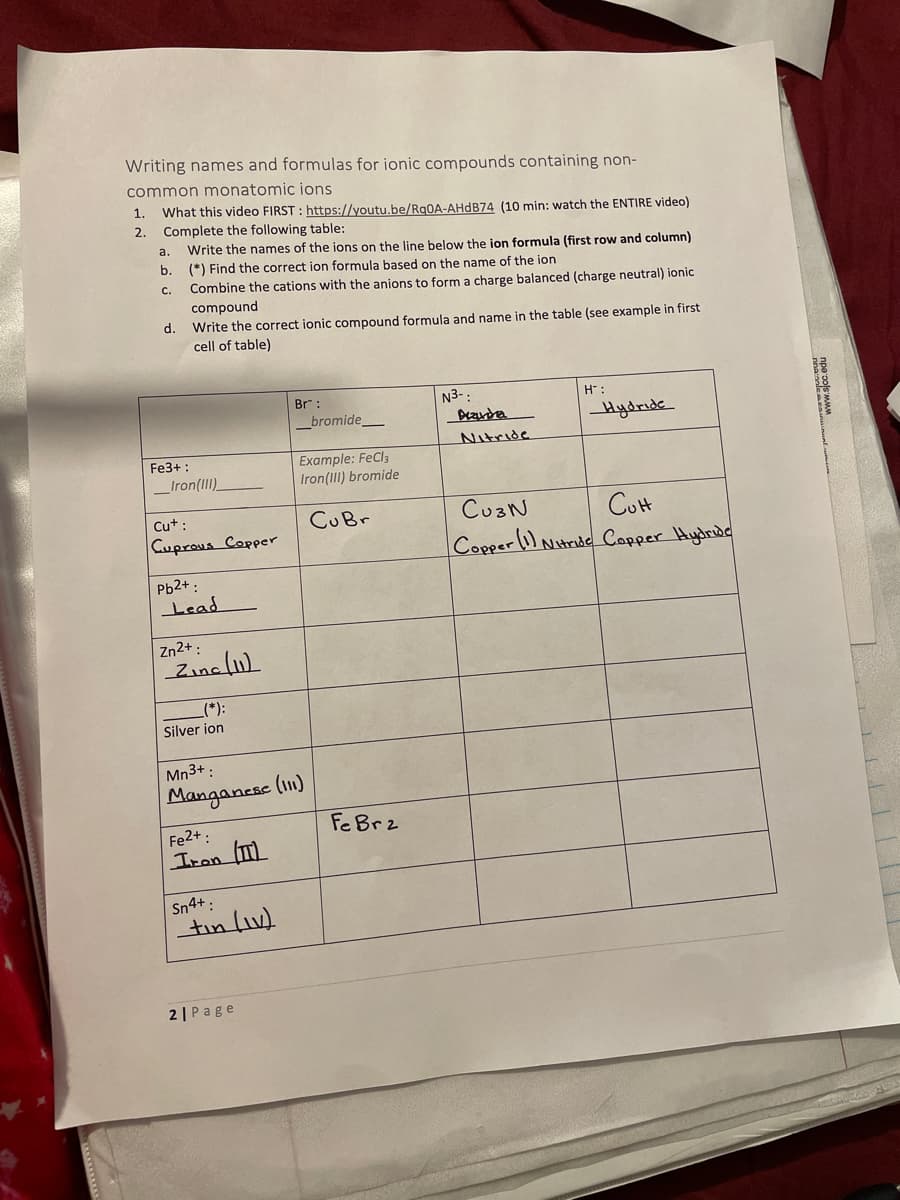
Transcribed Image Text:Writing names and formulas for ionic compounds containing non-
common monatomic ions
1.
What this video FIRST : https://youtu.be/RgOA-AHDB74 (10 min: watch the ENTIRE video)
2.
Complete the following table:
Write the names of the ions on the line below the ion formula (first row and column)
(*) Find the correct ion formula based on the name of the ion
a.
b.
C.
Combine the cations with the anions to form a charge balanced (charge neutral) ionic
compound
d.
Write the correct ionic compound formula and name in the table (see example in first
cell of table)
Br" :
N3-:
H :
_bromide_
Hydride
Nitride
Fe3+ :
Example: FeCl3
Iron(III) bromide
_Iron(III)
CuaN
CUH
Copper(i) Nitride Copper Hydride
Cut :
CUBr
Cuprous Copper
Pb2+ :
Lead
Zn2+ :
Zine lu)
_(*):
Silver ion
Mn3+ :
Manganese (1)
Fe Brz
Fe2+:
Iron (m
Sn4+:
tin lv)
2 | Page
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning
