wt. of empty flask with foil and wire, (g) Weight of empty flask (g) Weight of water (g) Density of water (g/mL) Temperature of boiling water (K) Volume of Erlenmeyer flask (mL) 123.98 123.67 (1) 0.99777 371.15 (2) Atmospheric pressure, (mm Hg) 758.35 Benzene TRIALS 1 3 Weight of flask with condensed vapor with foil and wire (g) Weight of condensed vapor (g) Molecular weight of benzene (g/mol) Mean Molecular Weight (g/mol) True Value (g/mol) % error 124.65 124.62 124.567 0.67 0.64 0.587 (3) (4) (5) (6) 78 (7) Chloroform TRIALS 1 2 3 Weight of flask with condensed vapor with foil and wire (g) Weight of condensed vapor (g) Molecular weight of chloroform (g/mol) Mean Molecular Weight (g/mol) True Value (g/mol) 125.0032 124.9954 125.0121 1.0232 1.0154 1.0321 (8) (9) (10) (11) 119.35 % error (12) Unknown Liquid TRIALS 1 2 3 Weight of flask with condensed vapor with foil and wire (g) Weight of condensed vapor (g) Molecular weight of unknown liquid (g/mol) 124.4865 124.4789 124.49 0.507 0.4989 0.51 (14) (16) 58 (17) (18-20) (13) (15) Mean Molecular Weight (g/mol) True Value (g/mol) % error What may this liquid be? (Clue: ketone)
wt. of empty flask with foil and wire, (g) Weight of empty flask (g) Weight of water (g) Density of water (g/mL) Temperature of boiling water (K) Volume of Erlenmeyer flask (mL) 123.98 123.67 (1) 0.99777 371.15 (2) Atmospheric pressure, (mm Hg) 758.35 Benzene TRIALS 1 3 Weight of flask with condensed vapor with foil and wire (g) Weight of condensed vapor (g) Molecular weight of benzene (g/mol) Mean Molecular Weight (g/mol) True Value (g/mol) % error 124.65 124.62 124.567 0.67 0.64 0.587 (3) (4) (5) (6) 78 (7) Chloroform TRIALS 1 2 3 Weight of flask with condensed vapor with foil and wire (g) Weight of condensed vapor (g) Molecular weight of chloroform (g/mol) Mean Molecular Weight (g/mol) True Value (g/mol) 125.0032 124.9954 125.0121 1.0232 1.0154 1.0321 (8) (9) (10) (11) 119.35 % error (12) Unknown Liquid TRIALS 1 2 3 Weight of flask with condensed vapor with foil and wire (g) Weight of condensed vapor (g) Molecular weight of unknown liquid (g/mol) 124.4865 124.4789 124.49 0.507 0.4989 0.51 (14) (16) 58 (17) (18-20) (13) (15) Mean Molecular Weight (g/mol) True Value (g/mol) % error What may this liquid be? (Clue: ketone)
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter16: Solubility And Precipitation Equilibria
Section: Chapter Questions
Problem 1P
Related questions
Question

Transcribed Image Text:Calculation of Molar Mass of Unknown Liquid
Using the Density Determined via Dumas
Method
Weight of flask filled with water (g)
385.76
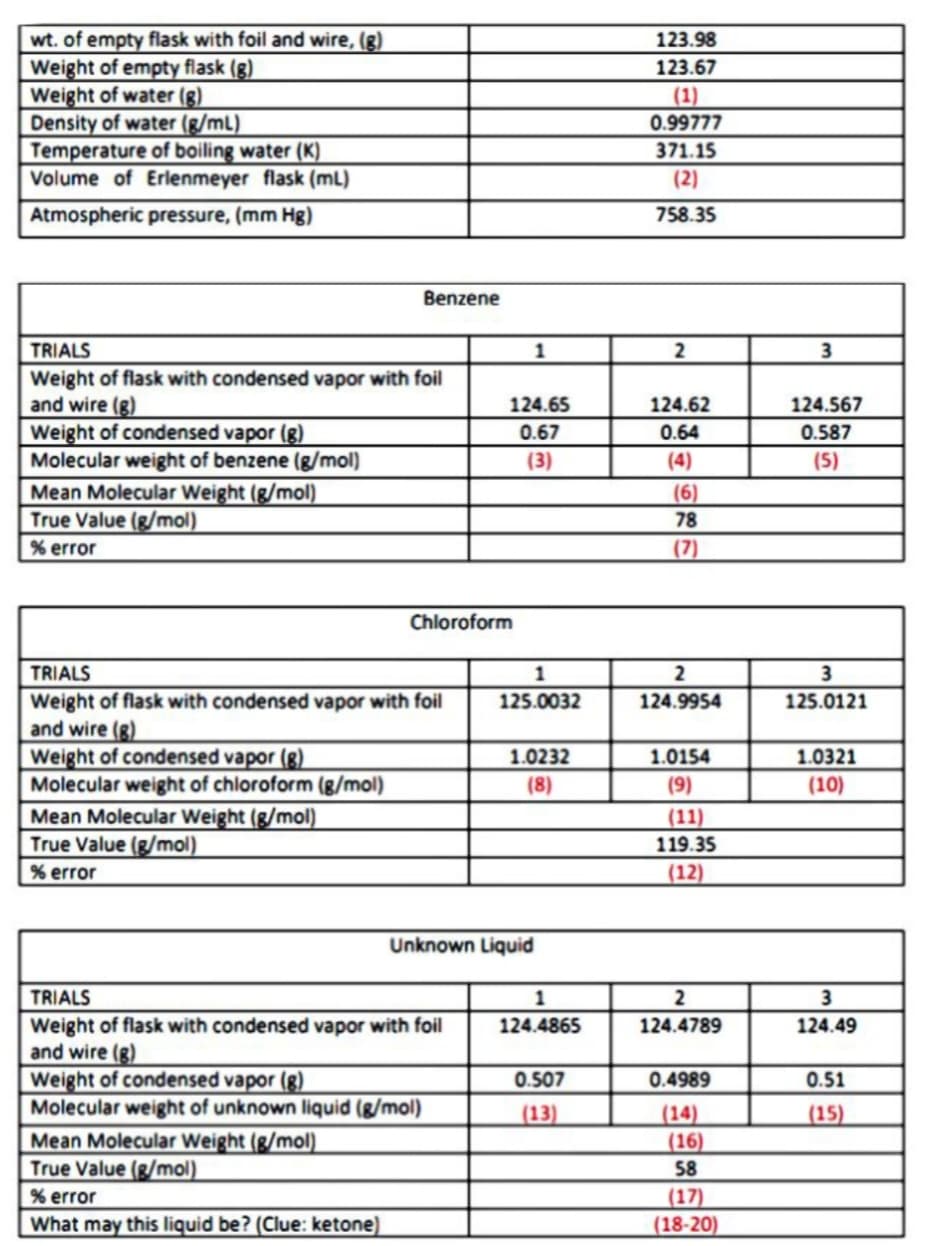
Transcribed Image Text:wt. of empty flask with foil and wire, (g)
Weight of empty flask (g)
Weight of water (g)
Density of water (g/mL)
Temperature of boiling water (K)
Volume of Erlenmeyer flask (ml)
123.98
123.67
(1)
ר0.9977
371.15
(2)
Atmospheric pressure, (mm Hg)
758.35
Benzene
TRIALS
1
3
Weight of flask with condensed vapor with foil
and wire (g)
Weight of condensed vapor (g)
Molecular weight of benzene (g/mol)
Mean Molecular Weight (g/mol)
True Value (g/mol)
124.65
124.62
124.567
0.67
0.64
0.587
(3)
(4)
(5)
(6)
78
% error
(7)
Chloroform
TRIALS
1
2
3
Weight of flask with condensed vapor with foil
and wire (8)
Weight of condensed vapor (g)
Molecular weight of chloroform (g/mol)
125.0032
124.9954
125.0121
1.0232
1.0154
1.0321
(8)
(9)
(10)
Mean Molecular Weight (g/mol)
True Value (g/mol)
(11)
119.35
% error
(12)
Unknown Liquid
TRIALS
1
2
Weight of flask with condensed vapor with foil
and wire (g)
Weight of condensed vapor (g)
Molecular weight of unknown liquid (g/mol)
124.4865
124.4789
124.49
0.507
0.4989
0.51
(13)
(14)
(16)
58
(15)
Mean Molecular Weight (g/mol)
True Value (g/mol)
% error
(17)
(18-20)
What may this liquid be? (Clue: ketone)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps

Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning


Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning


Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning