X bMy Questions | bartleby 101 Chem 101 X X app.101edu.co Submit Question 38 of 43 Write the balanced COMPLETE ionic equation for the reaction when Na2CO3 and AgNOs are mixed in aqueous solution. If no reaction occurs, write only NR. 4- |2- |2+ 3+ 4+ + 83 1 2 3 4 5 6 7 0 4 3 17 1 5 (g) (aq) (s) (1) NR Ag С N Na Reset Delete x H20 8:47 AM е OTYPE here to search 11/8/2019 S LO
X bMy Questions | bartleby 101 Chem 101 X X app.101edu.co Submit Question 38 of 43 Write the balanced COMPLETE ionic equation for the reaction when Na2CO3 and AgNOs are mixed in aqueous solution. If no reaction occurs, write only NR. 4- |2- |2+ 3+ 4+ + 83 1 2 3 4 5 6 7 0 4 3 17 1 5 (g) (aq) (s) (1) NR Ag С N Na Reset Delete x H20 8:47 AM е OTYPE here to search 11/8/2019 S LO
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter4: Reactions In Aqueous Solution
Section: Chapter Questions
Problem 57QAP: A sample of limestone weighing 1.005 g is dissolved in 75.00 mL of 0.2500 M hydrochloric acid. The...
Related questions
Question
Transcribed Image Text:X
bMy Questions | bartleby
101 Chem 101
X
X
app.101edu.co
Submit
Question 38 of 43
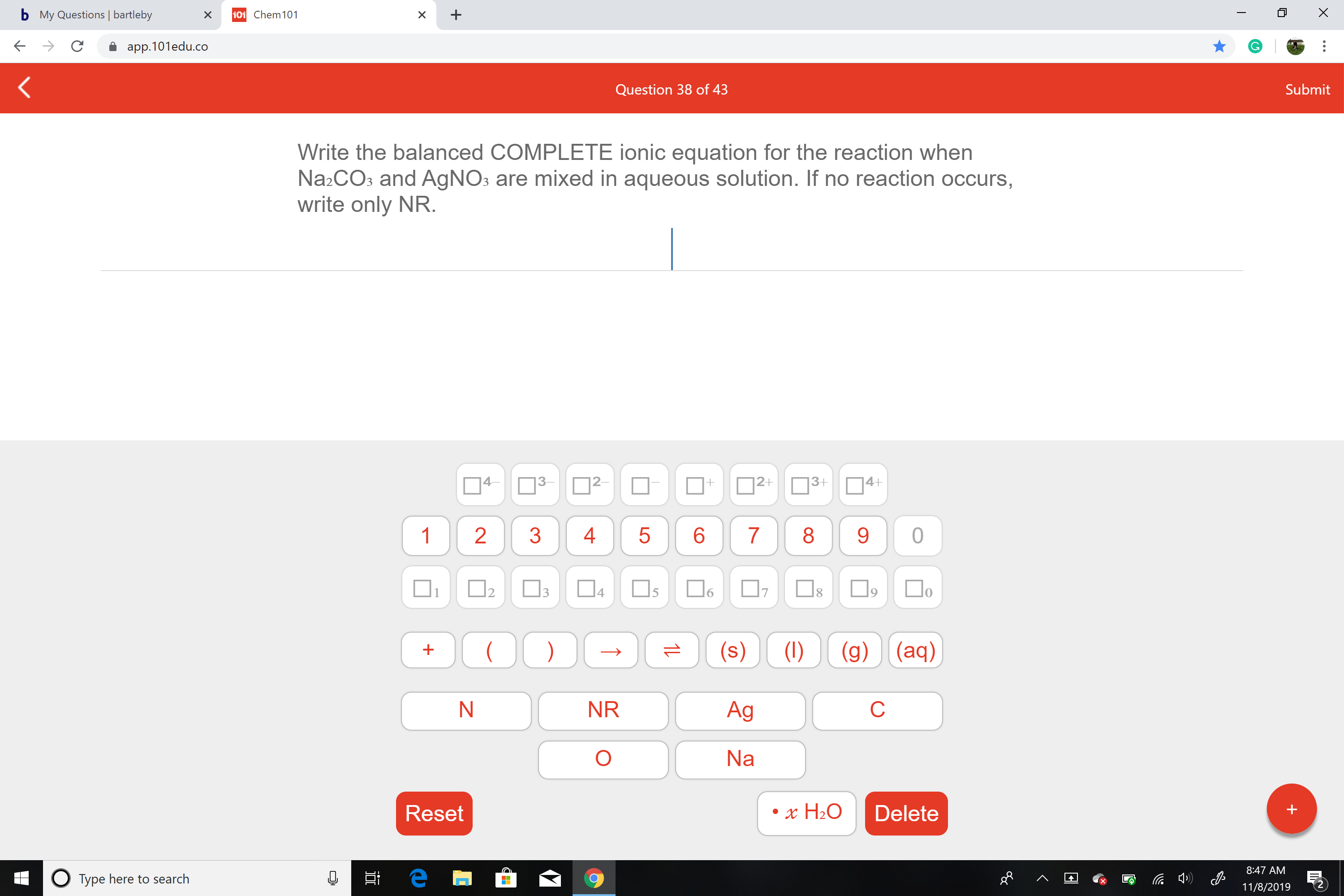
Write the balanced COMPLETE ionic equation for the reaction when
Na2CO3 and AgNOs are mixed in aqueous solution. If no reaction occurs,
write only NR.
4-
|2-
|2+
3+
4+
+
83
1
2
3
4
5
6
7
0
4
3
17
1
5
(g) (aq)
(s)
(1)
NR
Ag
С
N
Na
Reset
Delete
x H20
8:47 AM
е
OTYPE here to search
11/8/2019
S
LO
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning