Chemistry & Chemical Reactivity
9th Edition
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter5: Principles Of Chemical Reactivity: Energy And Chemical Reactions
Section: Chapter Questions
Problem 110SCQ: Peanuts and peanut oil are organic materials and bum in air. How many burning peanuts does it take...
Related questions
Question
2,3,4
Transcribed Image Text:ks
Surrealism-The M..
New folder
Genetic drift - Wikip.
- Algebra Foundation.
Image res
Morris - Specific Heat Practice ☆D O
t View Insert Format Tools Add-ons Help Accessibility
Last edit
New Rocker
BIUA
100% -
Normal text
22 +
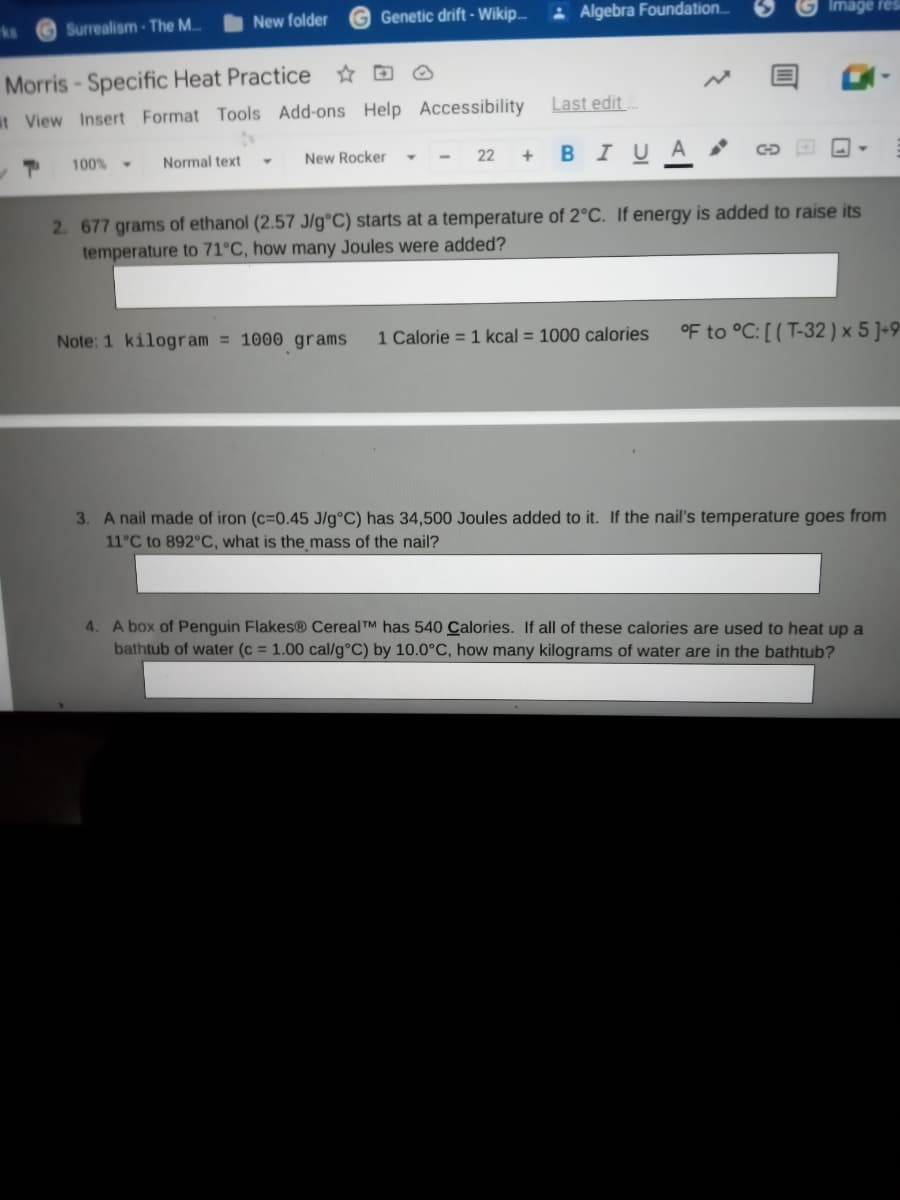
2. 677 grams of ethanol (2.57 J/g°C) starts at a temperature of 2°C. If energy is added to raise its
temperature to 71°C, how many Joules were added?
Note: 1 kilogram = 1000 grams
1 Calorie = 1 kcal = 1000 calories
OF to °C: [ ( T-32 ) x 5 J-9
3. A nail made of iron (c=0.45 J/g°C) has 34,500 Joules added to it. If the nail's temperature goes from
11°C to 892°C, what is the mass of the nail?
4. A box of Penguin Flakes® Cereal TM has 540 Calories. If all of these calories are used to heat up a
bathtub of water (c = 1.00 cal/g°C) by 10.0°C, how many kilograms of water are in the bathtub?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning