You are studying a liquid that you have just synthesized in the laboratory, and you would like to get a rough idea of its enthalpy of J vaporization. Assuming that it reflects Trouton's rule (namely that S≈ 88- .), you measure its normal boiling point and find that it is mol. K vap 368 K. Use this result to estimate AvapH in kJ/mol.
Q: - D1.2. A vector field S is expressed in rectangular coordinates as S = {125/ [(x-1)²+(y-2)² +…
A:
Q: 13). Show that the work required is 5.41k,Q"/s to place the same four charged particles of size at…
A: That the work required to assemble four identical charged particles of magnitude Q at the corners of…
Q: nydro-electric station has an out put or generate usoriok wh Per one year and consume / discharge)…
A: Three types of turbines are used based on the capacity of the water: 1.Francis, 2. Pelton…
Q: Find RMS velocity of three molecule having velocities 10 km/s, 20 km / s, 30 km/s.
A: We need to compute-RMS velocity (C2)=?The data given as-C1=10 km/sC2=20 km/sC3=30 km/s
Q: When a chronometer begins with a time of zero seconds, matching soccer balls have a horizontal…
A: We know the height of the ball. We also know that motion in perpendicular directions are independent…
Q: The equation of a stationary wave is TX given by Y = 6 cos sin 40 t cm. Which of the 5 following…
A:
Q: Suppose a point charge produces a potential of -1.7 V at a distance of 1.1 mm? What is the charge,…
A: Given: In this question, the given details are, Suppose a point produces a potential of -1.7 V at a…
Q: Find the solution to the following differential equation: d dy dx 2y x-y'
A:
Q: In many keyboards, the key switches are built as air-filled parallel-plate capacitors with a spacing…
A: The capacitance of parallel plate capacitor is given as C=Aεod Where A,d are plate area and…
Q: A man in a spaceship moving with a speed of 0.6c away from a space platform shines a light of…
A: Velocity of man u=0.6 c m/s. Wavelength λ=500 nm. Velocity of light c=3×108 m/s
Q: An archer shoots a 0.024-kg arrow at a target with a speed of 54 m/s. When it hits the target, it…
A: We are given the initial velocity. We are also given the stopping distance. We first find the…
Q: A system consisting of six indistinguishable particles obeys the Fermi-Dirac statistics. This system…
A: Given that there are 6 particles which follows Fermi-dirac statistics. Degeneracy of each level is…
Q: In introductory physics laboratories, a typical Cavendish balance for measuring the gravitational…
A: Gravity pulls an object towards it. It is a central force which brings 2 bodies towards each other.…
Q: SORES JE 7 носонт на котого он до пока он +1||острое+ лоз
A: The Bhor theory can be used to study atoms with one electron. When an atom gets ionized it will lose…
Q: 26. Find the tension in each of the three cables supporting the traffic light if it weighs 2.00 ×…
A:
Q: s from 0.61 in of the wi
A: Given: Wire's load increased from 3 to 5 Kg wt Elongation increased from 0.61 mm to 1.02 mm
Q: Group Problem: You are planning to build a log cabin in Northern Minnesota on a remote hill with a…
A: Given,Weight of the log = 1000 lbsAngle with espect to horizontal = θ =20°The coefficient of kinetic…
Q: : A 60 watt electric bulb loses its energy entirely by radiation from the surface of filament. If…
A: Solution:-Given thatEnergy lost by bulb by radiation (Q˙)=60 WSurface area of filament (A)=4…
Q: Please write down the proof of the Gay-Lussac-Joule experiment, i.e. Hint: you may use two-step…
A: The proof is given below:
Q: A geostationary satellite is orbiting the earth at a height of 6R above the surface of the earth…
A: We are aware that a geostationary satellite orbits the planet at a height of 6R above the surface,…
Q: 70 cal of heat are required to raise the temperature of 2 moles of an ideal diatomic gas at constant…
A: In order to raise the temperature of two moles of an ideal diatomic gas from 30 to 35 degrees…
Q: 3. A roller coaster is shown in the figure. Assuming no friction, calculate the speed at points B,…
A:
Q: In a band structure, a hole represents absence of an electron which results the mass of a hole to be…
A: Determine, The effective mass is negative for the hole.
Q: A piece of ice (heat capacity = 2100 Jkg^-1 C^-1 and latent heat = 3.36 x 10^5 J kg) of mass m gram…
A: Given that a mass of m gram of ice is -5oC at atmospheric pressure. It receives 420 J of heat in…
Q: The three positive charges lie at the vertices of an equilateral triangle, as shown in Figure P24.6.…
A:
Q: A water cooler of storage capacity 120 litres can cool water at a constant rate of P watts. In a…
A: We are aware that a 120-liter water cooler is capable of maintaining a constant P watts of…
Q: Calculate energy by a black body surface of area when it is maintained at 227° C. liven 5 =…
A: We need to compute-Energy radiated (Q)=?The data given as-Surface area (A)=100 cm2A=10-2…
Q: 21 (27). The lead wire bent in a semicircular shape is uniformly charged S with propaganda charge…
A: Given : Charge Density of wire : λ Radius of circular loop is R Concept : The potential due to…
Q: What is the frequency of a 4 cm wave traveling at 16 cm/s?
A: Solution:-Given thatWavelength of the wave (λ)=4 cmSpeed of wave (V)=16 cm/s
Q: [Q2] A resistor, an inductor, and a capacitor are connected in parallel to a sinusoidal source of…
A: There are 2 types of sources. They are dc or direct current source and ac or alternating current…
Q: Satellite feeds show that a wind has speed of 10 km/h going to the south. It will meet Habagat…
A: Given, Velocity of the wind moving south v1→ = 10 km/h (-j^) Speed of the wind moving 30 degree…
Q: A 1.10 kg sond, uniform ball of radius 0,140 m is released from rest at point A in the figure below,…
A:
Q: Deleumine value of univerrual. gas constant. If pressciue exented. by 4.0.32gm of hydungen occupying…
A: We have to compute-Value of universal gas constant (R)=?The data given as-Pressure (P)=2.703×105…
Q: 2. A homogeneous sheet metal part is given on the figure, determine the centre point of gravity.…
A: We are given a shape here. We divide the shape into 2 semicircles and a square. We know the center…
Q: In the quark model of fundamental particles, a proton is composed of three quarks: two "up" quarks,…
A: Part(a) Take the distance to be 1.14×10-15 m and calculate the potential energy of the subsystem of…
Q: S vii. V111. corresponding random speeds. Plot a graph of centripetal force, F, versus v². Find the…
A: Formula Used: 1) Slope = (Y2-Y1 )/(X2 - X1) where, Y2 and Y1 are Points on y axis and X2 and X1 are…
Q: There is a clever kitchen gadget for drying lettuce leaves after you wash them. It consists of a…
A: Angular speed of Cylinder ω= 2.45 revolution/s or Hz Radius of cylinder R= 12.2cm=.122m The…
Q: Calculate the radii of first and second permitted orbits of a hydrogen atom. Determine the values of…
A: Thus, the radius of nth permissible orbit for electron in hydrogen atom is given by rn=n2 h2 ∈0π m Z…
Q: An electric guitar's amplifier is at a distance of 4.7 m. Find the intensity of its sound waves when…
A:
Q: The Russian Spektr-R satellite was recently placed in an Earth orbit with an altitude at perigee of…
A: Part(a) What is the semi-major axis of the orbit. (b) What is the eccentricity. (c) If the true…
Q: What is the number of degrees of freedom of a unicycle? Note: we do not assume that it is on the…
A: Given : Degree of freedom M = 6N - K
Q: Q10. Calculate the maximum work obtained when 0.75 mol of an ideal gas expands isothermally and…
A: Given data, Number of moles n = 0.75 mol. Temperature T=270 C=273+27=300 K. Initial volume V1=15 L…
Q: 5. If the resultant force is required to act along the positive u axis and have a magnitude of 5 kN,…
A: Given: To find: Magnitude of Fb and it's direction theta
Q: 4. What is the percentage strength (v/v) if 225 g of a liquid having a specific gravity of 0.8 is…
A: We know that specific gravity is the density of liquid divided by density of water at 4 degree. We…
Q: What two properties of an electron did Robert Millikan determine from his experiments?
A: The famous experiment that Millikan performed is known as the "Millikan Oil Drop Experiment". For…
Q: A 10 kg cart is traveling at a velocity of 4 m/s in the rightward direction. It strikes a 7 kg cart…
A: Given that-Mass of the heavier cart, M=10 kgMass of the lighter cart, m= 7 kgInitial velocity of the…
Q: The San Juanico Bridge, which is the longest bridge in the Philippines spanning across a body of…
A: We are given the length of primary span. We know that solids expand on heating. This is called…
Q: x (a) Determine the electric potential at all points on the z-axis. Assume that the potential goes…
A: Solution: A disc of radius R is uniformly distributed with a positive charge. Given that the charge…
Q: An alpha particle reaches 28 fm in a gold foil experiment before it lost all of its kinetic. energy.…
A: Since the nucleus is positively charged it will repel any positive charge that gets close to it.…
Q: 1. Specify the axial relationship for each of the following three dimensional systems. (a) Trigonal…
A: Specify the axial relationship for each of the following three dimensional systems. (a) Trigonal (b)…
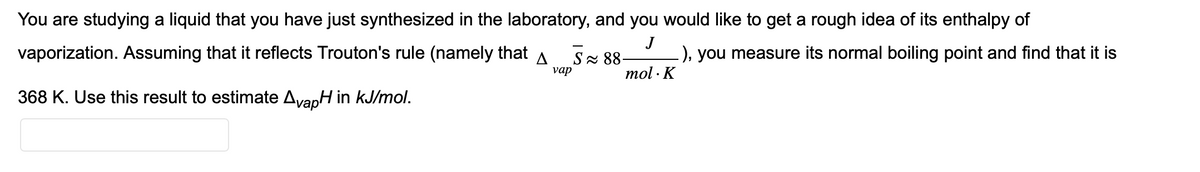
Step by step
Solved in 2 steps
