You have a 11.5 mg sample of blood that contains various proteins. Hemoglobin is the only protein in the sample containing Fe. Hemoglobin contains 3.83% by mass of a compound called heme (C34H32FeN4O4, MW = 616.49 g/mol). You find that your 11.5 mg blood sample contains 29.7 micrograms of Fe. What is the mass percent of hemoglobin in your protein sample?
You have a 11.5 mg sample of blood that contains various proteins. Hemoglobin is the only protein in the sample containing Fe. Hemoglobin contains 3.83% by mass of a compound called heme (C34H32FeN4O4, MW = 616.49 g/mol). You find that your 11.5 mg blood sample contains 29.7 micrograms of Fe. What is the mass percent of hemoglobin in your protein sample?
Living By Chemistry: First Edition Textbook
1st Edition
ISBN:9781559539418
Author:Angelica Stacy
Publisher:Angelica Stacy
ChapterU4: Toxins: Stoichiometry, Solution Chemistry, And Acids And Bases
SectionU4.7: Lethal Dose: Toxicity
Problem 4E
Related questions
Question
Transcribed Image Text:tab
caps lock
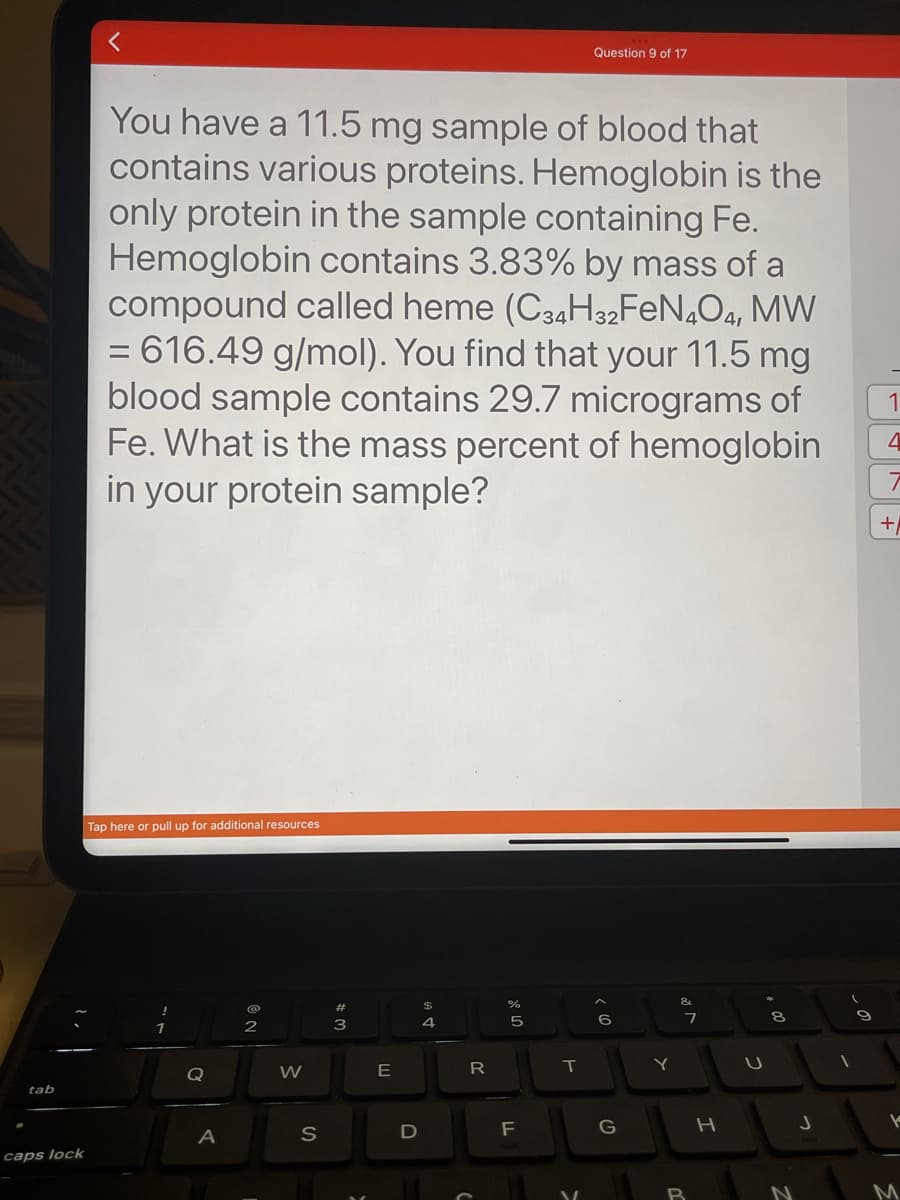
You have a 11.5 mg sample of blood that
contains various proteins. Hemoglobin is the
only protein in the sample containing Fe.
Hemoglobin contains 3.83% by mass of a
compound called heme (C34H32FeN4O4, MW
= 616.49 g/mol). You find that your 11.5 mg
blood sample contains 29.7 micrograms of
Fe. What is the mass percent of hemoglobin
in your protein sample?
Tap here or pull up for additional resources
Q
A
NO
2
W
S
#3
E
D
GA
4
R
%
5
F
Question 9 of 17
T
6
G
Y
&
7
B
H
8
N
9
4
+/
V
M
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:
9781559539418
Author:
Angelica Stacy
Publisher:
MAC HIGHER

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:
9781559539418
Author:
Angelica Stacy
Publisher:
MAC HIGHER

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning