You have a mixture of the following compounds dissolved in diethyl ether. Which aqueous system could be used to extract that compound from diethyl ether into the aqueous layer at room temperature?? | Write under each compound either: Water, 1M NaOH, 1M HCl or None (not extractable by aqueous system). COH کمه پلامه
You have a mixture of the following compounds dissolved in diethyl ether. Which aqueous system could be used to extract that compound from diethyl ether into the aqueous layer at room temperature?? | Write under each compound either: Water, 1M NaOH, 1M HCl or None (not extractable by aqueous system). COH کمه پلامه
Macroscale and Microscale Organic Experiments
7th Edition
ISBN:9781305577190
Author:Kenneth L. Williamson, Katherine M. Masters
Publisher:Kenneth L. Williamson, Katherine M. Masters
Chapter7: Extraction
Section: Chapter Questions
Problem 1Q
Related questions
Question
Please explain clearly so I can understand. Thank you!
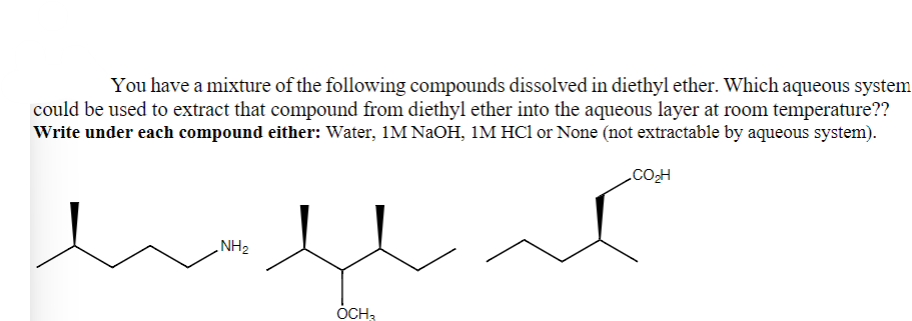
Transcribed Image Text:You have a mixture of the following compounds dissolved in diethyl ether. Which aqueous system
could be used to extract that compound from diethyl ether into the aqueous layer at room temperature??
Write under each compound either: Water, 1M NaOH, 1M HC1 or None (not extractable by aqueous system).
CO₂H
كريلام
OCH3
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Macroscale and Microscale Organic Experiments
Chemistry
ISBN:
9781305577190
Author:
Kenneth L. Williamson, Katherine M. Masters
Publisher:
Brooks Cole

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT

Macroscale and Microscale Organic Experiments
Chemistry
ISBN:
9781305577190
Author:
Kenneth L. Williamson, Katherine M. Masters
Publisher:
Brooks Cole

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT