You have carried out an experiment using the spectrophotometry concept. A solution containing compound X is mixed with reagent 1 and then reagent 2. This mixture produces a blue colour whose absorbance (A) could be read at 550 nm. The results are shown below If the standard solution (compound X) used have a concentration of 1 mM: 3. If the absorbance was read using 1 cm diameter cuvettes, calculate the molar extinction coefficient for compound X in the solution. State the unit for the coefficient. Assume each molecule of X produces one molecule of coloured compound
You have carried out an experiment using the spectrophotometry concept. A solution containing compound X is mixed with reagent 1 and then reagent 2. This mixture produces a blue colour whose absorbance (A) could be read at 550 nm. The results are shown below If the standard solution (compound X) used have a concentration of 1 mM: 3. If the absorbance was read using 1 cm diameter cuvettes, calculate the molar extinction coefficient for compound X in the solution. State the unit for the coefficient. Assume each molecule of X produces one molecule of coloured compound
Chapter14: Chromatography
Section: Chapter Questions
Problem 9P
Related questions
Question
You have carried out an experiment using the spectrophotometry concept. A solution
containing compound X is mixed with reagent 1 and then reagent 2. This mixture produces a
blue colour whose absorbance (A) could be read at 550 nm. The results are shown below
If the standard solution (compound X) used have a concentration of 1 mM:
3. If the absorbance was read using 1 cm diameter cuvettes, calculate the molar extinction
coefficient for compound X in the solution. State the unit for the coefficient. Assume
each molecule of X produces one molecule of coloured compound
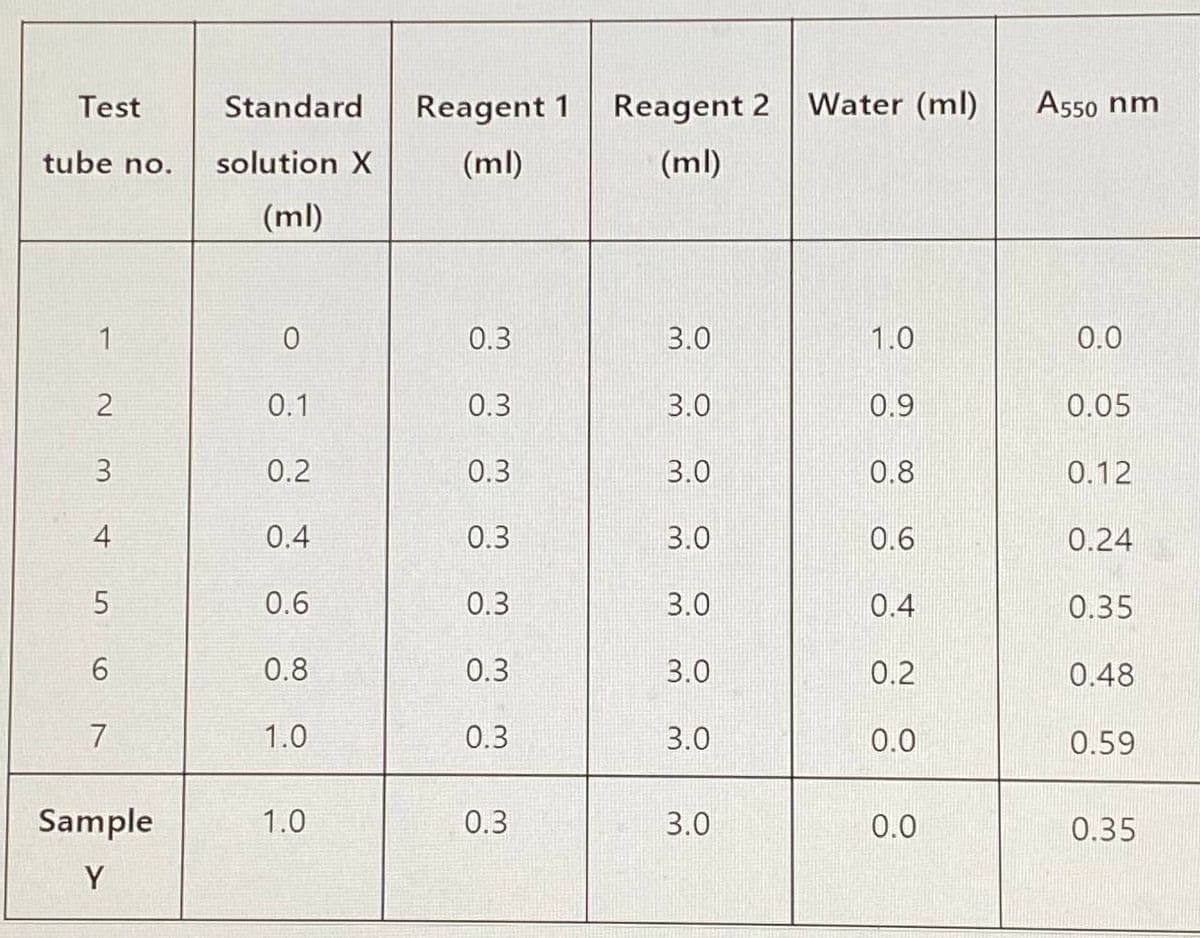
Transcribed Image Text:Test
tube no.
2
3
4
5
6
7
Sample
Y
Standard
solution X
(ml)
0
0.1
0.2
0.4
0.6
0.8
1.0
1.0
Reagent 1 Reagent 2
(ml)
(ml)
0.3
3.0
0.3
3.0
0.3
3.0
0.3
3.0
0.3
3.0
0.3
3.0
0.3
3.0
0.3
3.0
Water (ml)
1.0
0.9
0.8
0.6
0.4
0.2
0.0
0.0
A550 nm
0.0
0.05
0.12
0.24
0.35
0.48
0.59
0.35
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 4 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

