You performed a reaction in the lab using zinc and sulfuric acid. You produced 28.6 g of zinc sulfate. You calculated that you should have produced 32.9 g. What was your percent yield for this reaction? Use the paperclip button below to attach files. Student can enter max 3500 characters B U 四
You performed a reaction in the lab using zinc and sulfuric acid. You produced 28.6 g of zinc sulfate. You calculated that you should have produced 32.9 g. What was your percent yield for this reaction? Use the paperclip button below to attach files. Student can enter max 3500 characters B U 四
Mathematics For Machine Technology
8th Edition
ISBN:9781337798310
Author:Peterson, John.
Publisher:Peterson, John.
Chapter24: Percent Practical Applications
Section: Chapter Questions
Problem 35A
Related questions
Question
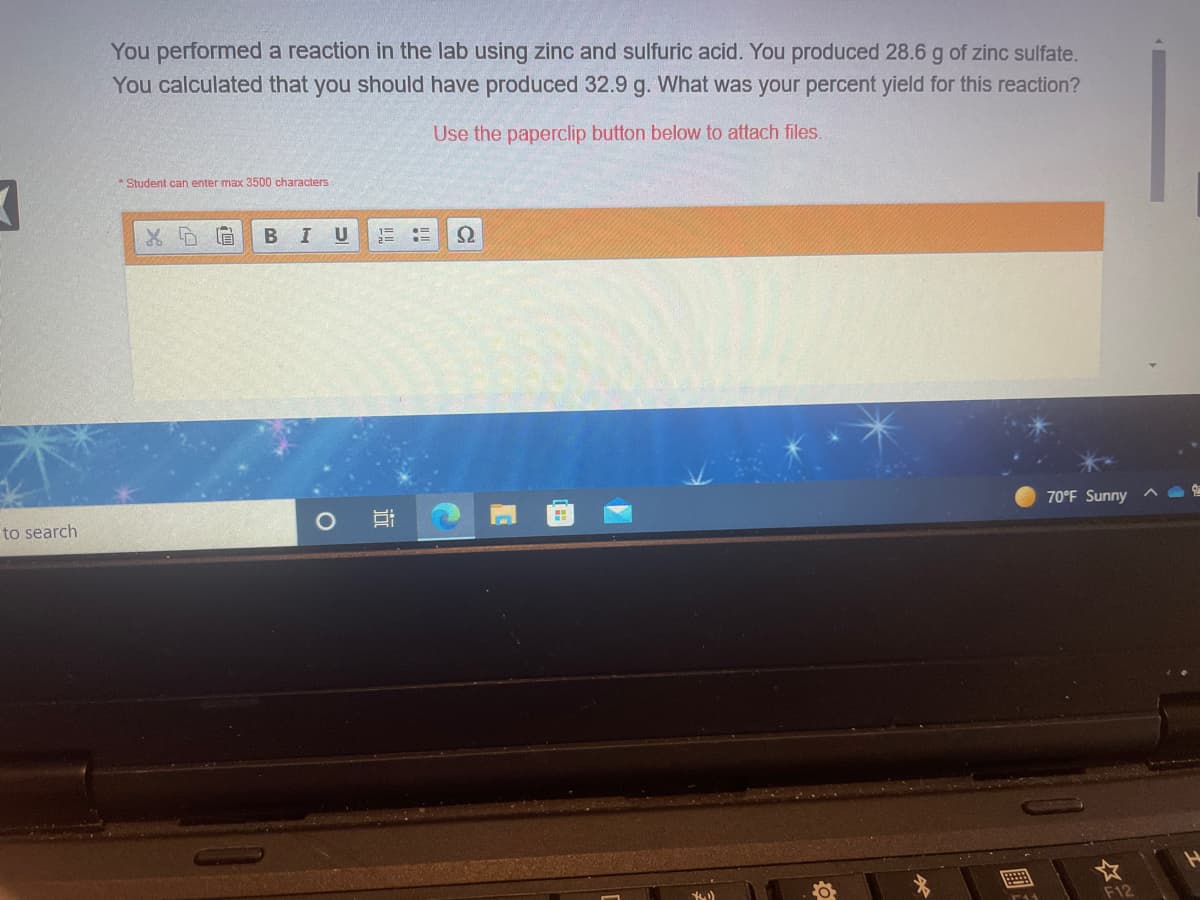
Transcribed Image Text:You performed a reaction in the lab using zinc and sulfuric acid. You produced 28.6 g of zinc sulfate.
You calculated that you should have produced 32.9 g. What was your percent yield for this reaction?
Use the paperclip button below to attach files.
* Student can enter max 3500 characters
В I
U
70°F Sunny
to search
F12
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Recommended textbooks for you

Mathematics For Machine Technology
Advanced Math
ISBN:
9781337798310
Author:
Peterson, John.
Publisher:
Cengage Learning,

Algebra: Structure And Method, Book 1
Algebra
ISBN:
9780395977224
Author:
Richard G. Brown, Mary P. Dolciani, Robert H. Sorgenfrey, William L. Cole
Publisher:
McDougal Littell

Mathematics For Machine Technology
Advanced Math
ISBN:
9781337798310
Author:
Peterson, John.
Publisher:
Cengage Learning,

Algebra: Structure And Method, Book 1
Algebra
ISBN:
9780395977224
Author:
Richard G. Brown, Mary P. Dolciani, Robert H. Sorgenfrey, William L. Cole
Publisher:
McDougal Littell