Chapter27: Biomolecules: Lipids
Section27.SE: Something Extra
Problem 17AP
Related questions
Question
IUPAC name for the following carboxylic acids
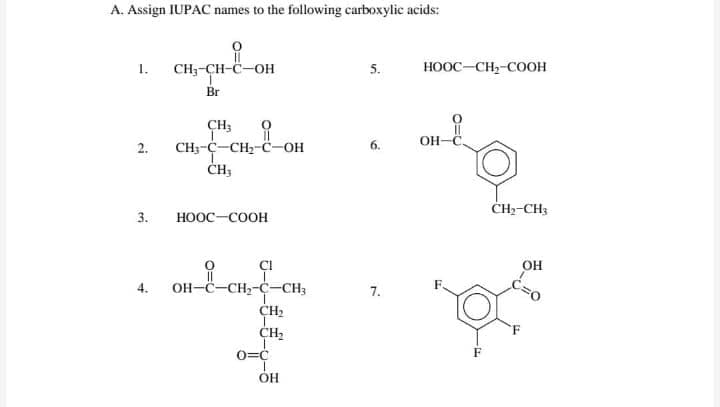
Transcribed Image Text:A. Assign IUPAC names to the following carboxylic acids:
1.
CH;-CH-C-OH
5.
HOOC-CH,-COOH
Br
CH3
OH
он-
2.
CH;-C-CH;-C-OH
CH-CH3
3.
ноос-соон
of
CI
OH
4.
OH-C-CH2-C-CH3
7.
CH2
CH2
0=C
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

