Answer – As per Boyle’s law, the pressure of a gas is inversely proportional to its volume.
Explanation:
Boyle’s law states that at a constant temperature, increasing the pressure on a gas decreases its volume and vice versa.
Mathematically, this relationship can be expressed as PV = constant or V = constant / P, where P represents the pressure and V represents the volume of the gas. This inverse relationship is graphically represented by a hyperbola on a pressure-volume graph.
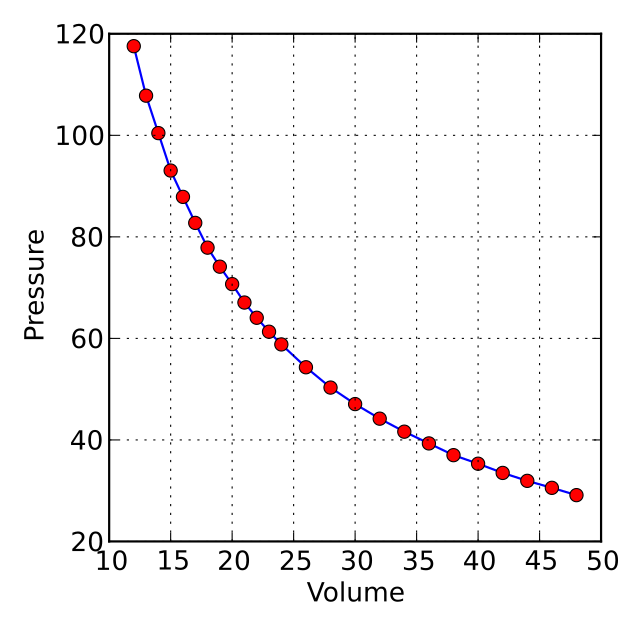
Image credit: Krishnavedala / Wikimedia Commons (licensed under CC BY-SA 3.0)
As seen in the graph above, it is evident that the relationship between these two variables is an inverse proportionality i.e. when one variable is increased, the other one decreases.