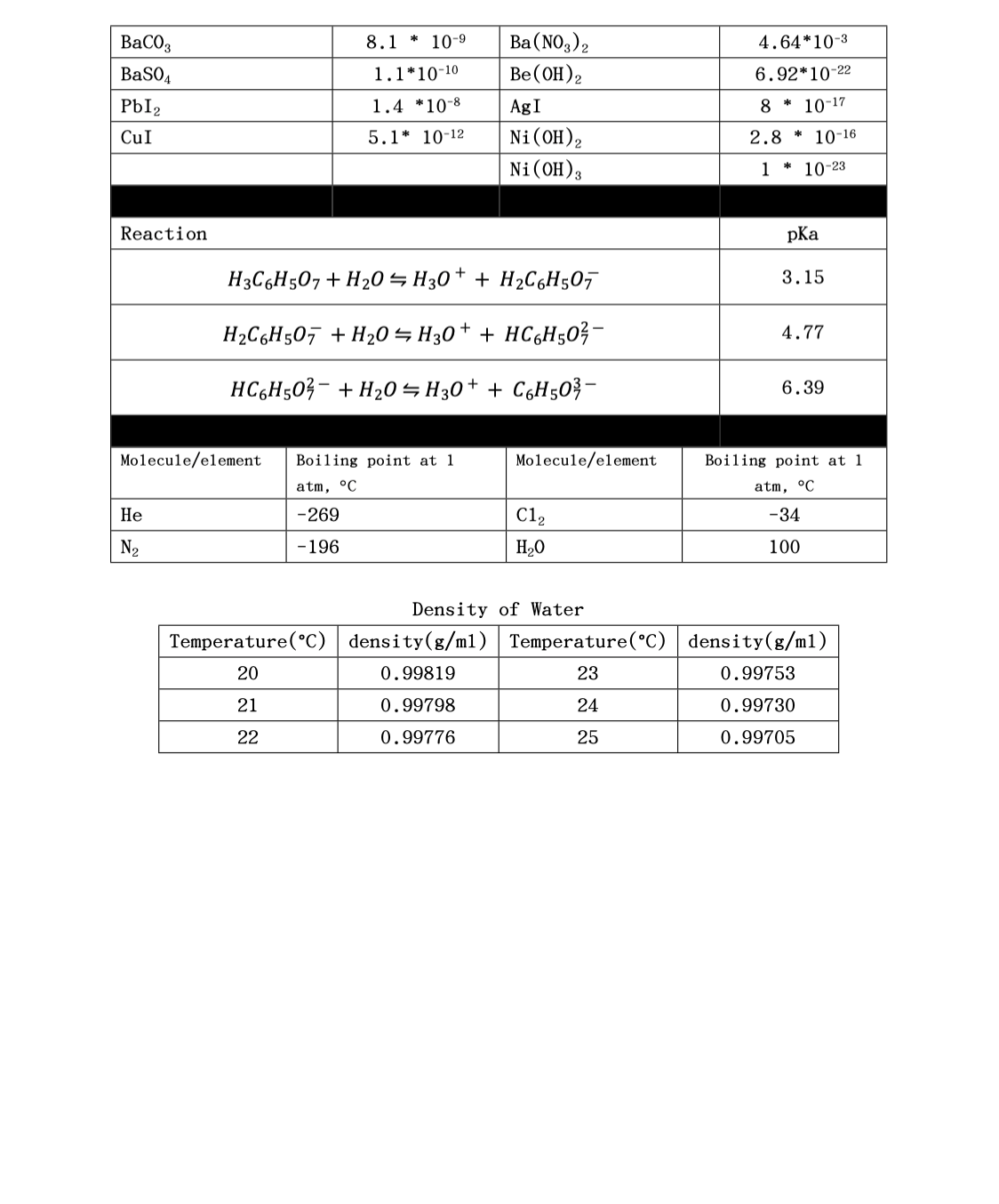
. 1.250 L of a 0.7601 mol/L Sodium Sulfide aqueous solution is mixed with 500.0 mL of a 0.7450mol/L aqueous solution of Lead (II) Nitrate. A. How much energy is absorbed or released by the reaction? B. If the reaction is carried out in a calorimeter whose constant is 14.60 kJ/°C and the solutions are initially at 21.000°C, what is the final temperature of the reaction solution on completion. Assume the heat capacity of the final solution is 4.200 J/g°C and that it has a density of 1.05 g/mL. Also assume that any solid formed does not absorb or give off heat.
Thermochemistry
Thermochemistry can be considered as a branch of thermodynamics that deals with the connections between warmth, work, and various types of energy, formed because of different synthetic and actual cycles. Thermochemistry describes the energy changes that occur as a result of reactions or chemical changes in a substance.
Exergonic Reaction
The term exergonic is derived from the Greek word in which ‘ergon’ means work and exergonic means ‘work outside’. Exergonic reactions releases work energy. Exergonic reactions are different from exothermic reactions, the one that releases only heat energy during the course of the reaction. So, exothermic reaction is one type of exergonic reaction. Exergonic reaction releases work energy in different forms like heat, light or sound. For example, a glow stick releases light making that an exergonic reaction and not an exothermic reaction since no heat is released. Even endothermic reactions at very high temperature are exergonic.
8. 1.250 L of a 0.7601 mol/L Sodium Sulfide aqueous solution is mixed with 500.0 mL of a 0.7450mol/L aqueous solution of Lead (II) Nitrate.
A. How much energy is absorbed or released by the reaction?
B. If the reaction is carried out in a calorimeter whose constant is 14.60 kJ/°C and the solutions are initially at 21.000°C, what is the final temperature of the reaction solution on completion. Assume the heat capacity of the final solution is 4.200 J/g°C and that it has a density of 1.05 g/mL. Also assume that any solid formed does not absorb or give off heat.
![AH( 1
(K2
In
K1
1
[A] = [A]o - kt r=k
= (RT)A"K.
R (T1
аА + bB cС + dD,
AG, = AG? + RT InQ
-b + b? – 4ac
X =
([A¯]
Ereduction
pH = pKa+ log
|[HA])
cell
+ Eoxidation
2а
pH =
-log([H*])
Ecell = Ecel1°
(RT ln Q)/nF if T =298.15 K then Ecell = Ecel1°
(0.025693V( 1n Q)/n)
In K = nF Ecel1°/RT if T = 298.15 K then In K = n
AG° = -nF Ecel1°
Ecel1/0.025693V
Some useful data
Element/Ion/Molecu
AH?, kJ/mol
Element/Ion/Molecu
AH?, kJ/mol
le
le
Na*(aq)
-240.12
NO, (aq)
-205.0
Pb2* (aq)
-1.7
PBSO,(s)
-919.94
I- (aq)
-55.19
CH;COO (aq)
-486.0
NH,*(aq)
S2- (aq)
-132.51
PbS (s)
-98.32
+33.1
Element
m, g/mol
Element
т, s/mo1
Ва
137.327
H
1.0079
S
32.065
C1
35.453
12.0107
15.9994
Element/Ion/Molecu
pKa
Element/Ion/Molecu
pKa
le
le
H3PO4
2.12
H,PO,
7.21
HCN
9.31
HPO,2-
12.68
HSO,
1.92
NH,*
9.25
CH3COOH
4.75
HCO3
10.25
HI
-10(estimate)
H2CO3
6.37
HIO3
0.77
HNO,
3.37
Molecule
Ksp
Molecule
Ksp
2|P age](/v2/_next/image?url=https%3A%2F%2Fcontent.bartleby.com%2Fqna-images%2Fquestion%2F5706d863-7215-45e2-86b7-e82f6e863746%2Fca5e12fc-fd7d-48b9-818a-e4b063305993%2F7d7uo6_processed.png&w=3840&q=75)
Step by step
Solved in 4 steps with 3 images







