. Balance the redox reactions by following the steps in the text. Rotate through the group, having each group member do the next step in the process and explain that step to the rest of the group. a. 1(s) + Fe(s) - Felz(s) b. Cl2(8) + H2Oz(aq) c. Hg*(aq) + H2(8) d. CH,OH(I) + O,(8) CI (aq) + O2(8) (acidic) Hg(1) + H2O(1) (basic) CO-(g) + H20(1) (acidic)
. Balance the redox reactions by following the steps in the text. Rotate through the group, having each group member do the next step in the process and explain that step to the rest of the group. a. 1(s) + Fe(s) - Felz(s) b. Cl2(8) + H2Oz(aq) c. Hg*(aq) + H2(8) d. CH,OH(I) + O,(8) CI (aq) + O2(8) (acidic) Hg(1) + H2O(1) (basic) CO-(g) + H20(1) (acidic)
Chemistry by OpenStax (2015-05-04)
1st Edition
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Chapter4: Stoichiometry Of Chemical Reactions
Section: Chapter Questions
Problem 41E: Balance each of the following equations according to the half-reaction method: (a)...
Related questions
Question
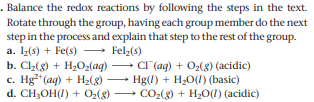
Transcribed Image Text:. Balance the redox reactions by following the steps in the text.
Rotate through the group, having each group member do the next
step in the process and explain that step to the rest of the group.
a. 1(s) + Fe(s) - Felz(s)
b. Cl2(8) + H2Oz(aq)
c. Hg*(aq) + H2(8)
d. CH,OH(I) + O,(8)
CI (aq) + O2(8) (acidic)
Hg(1) + H2O(1) (basic)
CO-(g) + H20(1) (acidic)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 12 images

Recommended textbooks for you

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax


Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax


Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning