● . Electrons scattered throughout . (a) Expected Results Diffuse positive charge Assuming that the arrows are streams of "alpha" radiation Use the image to determine whether the "alpha radiation" is positively or negatively charged. Explain how you arrived at your conclusion. Cite evidence from the image(s) in your explanation. Path of a particle (b) Actual Results
● . Electrons scattered throughout . (a) Expected Results Diffuse positive charge Assuming that the arrows are streams of "alpha" radiation Use the image to determine whether the "alpha radiation" is positively or negatively charged. Explain how you arrived at your conclusion. Cite evidence from the image(s) in your explanation. Path of a particle (b) Actual Results
Introductory Chemistry: An Active Learning Approach
6th Edition
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Mark S. Cracolice, Ed Peters
Chapter11: Atomic Theory :the Quantum Model Of The Atom
Section: Chapter Questions
Problem 103E
Related questions
Question
100%
Cite evidence from the images.
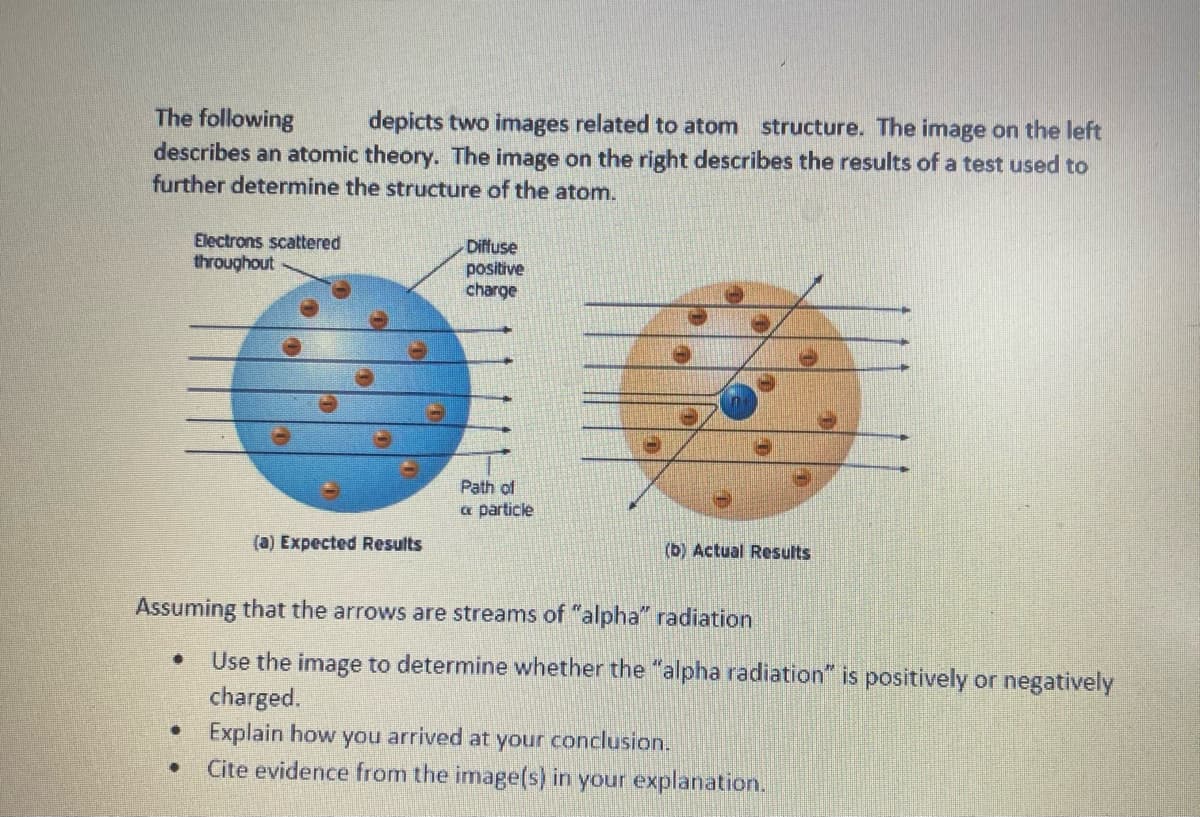
Transcribed Image Text:The following
depicts two images related to atom structure. The image on the left
describes an atomic theory. The image on the right describes the results of a test used to
further determine the structure of the atom.
D
.
Electrons scattered
throughout
●
(a) Expected Results
Diffuse
positive
charge
Assuming that the arrows are streams of "alpha" radiation
Use the image to determine whether the "alpha radiation" is positively or negatively
charged.
Explain how you arrived at your conclusion.
Cite evidence from the image(s) in your explanation.
Path of
& particle
(b) Actual Results
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning