. Figure 8–59 illustrates an H,O molecule. The O-H bond length is 0.096 nm and the H- 0-H bonds make an angle of 104°. Calculate the moment of inertia of the H2O molecule (assume the atoms are points) about an axis passing through the center of the oxygen atom (a) perpendicular to the plane of the molecule, and (b) in the plane of the molecule, bisecting the H-0-Hbonds. H |104° FIGURE 8–59 H Problem 82.
. Figure 8–59 illustrates an H,O molecule. The O-H bond length is 0.096 nm and the H- 0-H bonds make an angle of 104°. Calculate the moment of inertia of the H2O molecule (assume the atoms are points) about an axis passing through the center of the oxygen atom (a) perpendicular to the plane of the molecule, and (b) in the plane of the molecule, bisecting the H-0-Hbonds. H |104° FIGURE 8–59 H Problem 82.
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter6: Quantum Mechanics And Molecular Structure
Section: Chapter Questions
Problem 66AP: Calcium carbide (CaC2) is an intermediate in the manufacturing of acetylene (C2H2) . It is the...
Related questions
Question
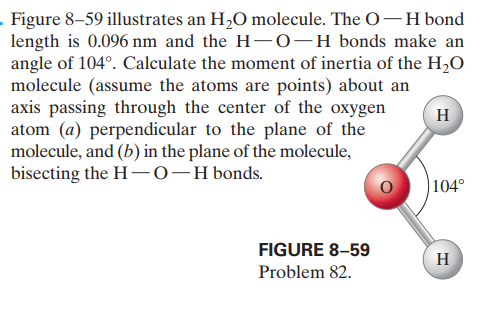
Transcribed Image Text:. Figure 8–59 illustrates an H,O molecule. The O-H bond
length is 0.096 nm and the H- 0-H bonds make an
angle of 104°. Calculate the moment of inertia of the H2O
molecule (assume the atoms are points) about an
axis passing through the center of the oxygen
atom (a) perpendicular to the plane of the
molecule, and (b) in the plane of the molecule,
bisecting the H-0-Hbonds.
H
|104°
FIGURE 8–59
H
Problem 82.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning