The oxygen atoms thus formed relax from the excited singlet configuration ('O') to the ground state triplet configuration (°O) by colliding with another molecule (such as N2) and transferring energy, analogous to a billiard ball collision. Once formed, ozone (03) is produced by collision of 3O with another O2 molecule: hv N, (or other molecule) + '0': • + O2 Оз hv Overall: 302 203 Recall that O3 is a 1,3 dipole with the following resonance representation: Ozone thus formed is normally destroyed by a combined thermal and photochemical route: hv O2 + O Оз + О 202 If the average bond strength of one of the bonds in O3 is 445 kJ/mol, what wavelength (A) and range of radiation is this (see table and formulae for question 1)?
The oxygen atoms thus formed relax from the excited singlet configuration ('O') to the ground state triplet configuration (°O) by colliding with another molecule (such as N2) and transferring energy, analogous to a billiard ball collision. Once formed, ozone (03) is produced by collision of 3O with another O2 molecule: hv N, (or other molecule) + '0': • + O2 Оз hv Overall: 302 203 Recall that O3 is a 1,3 dipole with the following resonance representation: Ozone thus formed is normally destroyed by a combined thermal and photochemical route: hv O2 + O Оз + О 202 If the average bond strength of one of the bonds in O3 is 445 kJ/mol, what wavelength (A) and range of radiation is this (see table and formulae for question 1)?
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter20: Molecular Spectroscopy And Photochemistry
Section: Chapter Questions
Problem 3P
Related questions
Question
Help
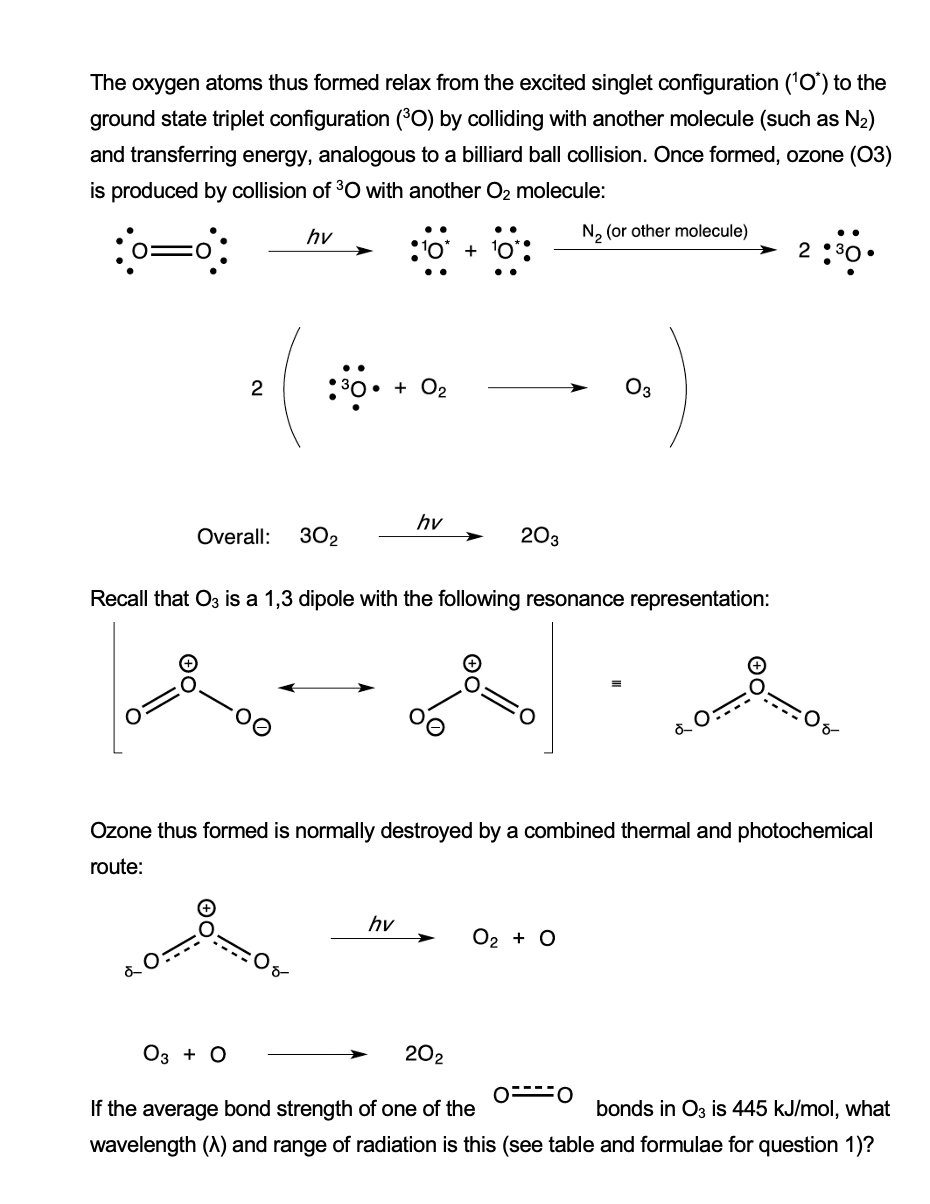
Transcribed Image Text:The oxygen atoms thus formed relax from the excited singlet configuration ('O) to the
ground state triplet configuration (°O) by colliding with another molecule (such as N2)
and transferring energy, analogous to a billiard ball collision. Once formed, ozone (03)
is produced by collision of 30 with another O2 molecule:
hy
N2 (or other molecule)
:'o* + 10
2 :30
2
30• + O2
Оз
hv
Overall:
302
203
Recall that O3 is a 1,3 dipole with the following resonance representation:
Ozone thus formed is normally destroyed by a combined thermal and photochemical
route:
hv
О2 + O
Оз + о
202
If the average bond strength of one of the
bonds in O3 is 445 kJ/mol, what
wavelength (A) and range of radiation is this (see table and formulae for question 1)?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning


Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning